122 Structure and Bonding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cr7ni Wheels: Supramolecular Tectons for the Physical Implementation of Quantum Information Processing
magnetochemistry Review Cr7Ni Wheels: Supramolecular Tectons for the Physical Implementation of Quantum Information Processing Jesus Ferrando-Soria † School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK; [email protected]; Tel.: +34-963-544460 † Present address: Instituto de Ciencia Molecular (ICMol), Universitat de València, Paterna 46980, València, Spain. Academic Editor: Floriana Tuna Received: 11 August 2016; Accepted: 15 September 2016; Published: 21 September 2016 Abstract: The physical implementation of quantum information processing (QIP) is an emerging field that requires finding a suitable candidate as a quantum bit (qubit), the basic unit for quantum information, which can be organised in a scalable manner to implement quantum gates (QGs) capable of performing computational tasks. Supramolecular chemistry offers a wide range of chemical tools to bring together, with great control, different molecular building blocks in order to grow supramolecular assemblies that have the potential to achieve the current milestones in the field. In this review, we are particularly interested in the latest research developments on the supramolecular chemistry approach to QIP using {Cr7Ni} wheels as qubits for the physical implementation of QGs. Special emphasis will be given to the unique high degree of chemical tunability of this unique class of heterobimetallic octanuclear rings, which results in an attractive playground to generate aesthetically pleasing supramolecular assemblies of increasing structural complexity and interesting physical properties for quantum computing. Keywords: {Cr7Ni} heterobimetallic rings; quantum information processing; supramolecular chemistry; qubits; quantum gates 1. Introduction: Molecules as Qubits The physical implementation of quantum information processing (QIP) is currently a subject of intense research in chemistry, physics, materials science, and nanotechnology because of the thrilling potential technological applications that chemical and physical systems may exhibit in quantum computing [1–4]. -

The 45Th Annual International Meeting of the ESR Spectroscopy Group of the Royal Society of Chemistry
The 45th Annual International Meeting of the ESR Spectroscopy Group of the Royal Society of Chemistry The University of Manchester 25th – 29th March 2012 Contents Conference Programme 3 Information for delegates 6 Getting there 6 Map of conference venue 9 University of Manchester campus map 10 Speaker/poster presenter information 11 Internet access 11 Car parking/taxis 11 Checking out and left luggage 11 Accompanying persons 12 Free afternoon 12 Manchester city centre map 13 Conference sponsors 14 EPR @ Manchester 15 Bruker prize lecture and reception 16 JEOL student prize lectures 17 Committee of the ESR spectroscopy Group of the RSC 18 Next meeting (2013) 19 Abstracts for Talks T1‐T48 Abstracts for Posters P1‐P31 Presenting Author Index R1‐R2 Title Index R3‐R6 List of participants R7‐R14 2 Conference Programme Sunday 25th March 16.00 – 18.30 Registration Chancellors Reception 18.30 – 20.00 Dinner Chancellors Carriage Restaurant RSC Wine Reception 20.00 – 22.30 Chancellors Conservatory and bar and free bar Monday 26th March 07.30 – 08.55 Breakfast Chancellors or Luther King House or Willowbank Hotel Session 1 Chair: David Collison 08.55 – 09.00 Mark Newton Conference opening and welcome note 09.00 – 09.30 Richard Winpenny Keynote Lecture: EPR Studies of Rings and Dimers of Rings Intercluster exchange interactions and spin state switching in 09.35 – 09.50 Irina Drozdyuk copper nitroxide based molecular magnets Cu(hfac)2LR studied by EPR Quantum operations by pulsed ESR spectroscopy: Molecular 09.55 – 10.10 Shigeaki Nakazawa design for -

A Monometallic Lanthanide Bis(Methanediide) Single Molecule Magnet with a Large Energy Barrier Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue A monometallic lanthanide bis(methanediide) single molecule magnet with a large energy barrier Cite this: Chem. Sci.,2016,7,155 and complex spin relaxation behaviour† Matthew Gregson,‡a Nicholas F. Chilton,‡a Ana-Maria Ariciu,b Floriana Tuna,b Iain F. Crowe,c William Lewis,d Alexander J. Blake,d David Collison,a Eric J. L. McInnes,b Richard E. P. Winpenny*a and Stephen T. Liddle*a We report a dysprosium(III) bis(methanediide) single molecule magnet (SMM) where stabilisation of the highly magnetic states and suppression of mixing of opposite magnetic projections is imposed by a linear arrangement of negatively-charged donor atoms supported by weak neutral donors. Treatment TMS TMS TMS 2À TMS of [Ln(BIPM )(BIPM H)] [Ln ¼ Dy, 1Dy;Y,1Y; BIPM ¼ {C(PPh2NSiMe3)2} ; BIPM H ¼ À TMS {HC(PPh2NSiMe3)2} ] with benzyl potassium/18-crown-6 ether (18C6) in THF afforded [Ln(BIPM )2] [K(18C6)(THF)2] [Ln ¼ Dy, 2Dy;Y,2Y]. AC magnetic measurements of 2Dy in zero DC field show Creative Commons Attribution 3.0 Unported Licence. temperature- and frequency-dependent SMM behaviour. Orbach relaxation dominates at high temperature, but at lower temperatures a second-order Raman process dominates. Complex 2Dy exhibits two thermally activated energy barriers (Ueff) of 721 and 813 K, the largest Ueff values for any monometallic dysprosium(III) complex. Dilution experiments confirm the molecular origin of this phenomenon. Complex 2Dy has rich magnetic dynamics; field-cooled (FC)/zero-field cooled (ZFC) susceptibility measurements show a clear divergence at 16 K, meaning the magnetic observables are out-of-equilibrium below this temperature, however the maximum in ZFC, which conventionally defines the blocking temperature, TB, is found at 10 K. -

Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation
doi.org/10.26434/chemrxiv.7067669.v1 Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation Marcus J. Giansiracusa, Andreas Kostopoulos, George F. S. Whitehead, David Collison, Floriana Tuna, Richard Winpenny, Nicholas Chilton Submitted date: 10/09/2018 • Posted date: 11/09/2018 Licence: CC BY-NC-ND 4.0 Citation information: Giansiracusa, Marcus J.; Kostopoulos, Andreas; F. S. Whitehead, George; Collison, David; Tuna, Floriana; Winpenny, Richard; et al. (2018): Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation. ChemRxiv. Preprint. We report a six coordinate DyIII single-molecule magnet (SMM) with an energy barrier of 1110 K for thermal relaxation of magnetization. The sample shows no retention of magnetization even at 2 K and this led us to find a good correlation between the blocking temperature and the Raman relaxation regime for SMMs. The key parameter is the relaxation time (ᵰ ) at the point where switch the Raman relaxation mechanism becomes more important than Orbach. File list (1) Dy-mon_final_for ChemRxiv.pdf (1.77 MiB) view on ChemRxiv download file Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation Marcus J. Giansiracusa, Susan Al-Badran, Andreas K. Kostopoulos, George F. S. Whitehead, David Collison, Floriana Tuna, Richard E. P. Winpenny*, and Nicholas F. Chilton* Dedications Abstract: We report a six coordinate DyIII single-molecule magnet length to the anionic DiMeQ oxygen donors at 2.150(4) Å. The (SMM) with an energy barrier of 1110 K for thermal relaxation of trans equatorial Dy-Cl bonds are 2.681(2) Å, and the third Cl magnetization. The sample shows no retention of magnetization even ligand trans to the neutral water ligand (2.32(1) Å) has a bond at 2 K and this led us to find a good correlation between the blocking length of 2.897(8) Å. -

James Walsh Postdoctoral Fellow Department of Chemistry
James Walsh Postdoctoral Fellow Department of Chemistry Northwestern University Evanston, IL 60208 phone: (847) 491-4356 email: [email protected] Current Position Assistant Professor, Department of Chemistry, University of Massachusetts Amherst (Sep 2019) Postdoctoral Fellow, Department of Chemistry, Northwestern University (2015 – Present) Advisors: Prof. Danna Freedman and Prof. Steven Jacobsen Background Postdoctoral Fellow, Aarhus University (2015) Advisor: Dr. Jacob Overgaard Ph.D. in Inorganic Chemistry, University of Manchester (2010 – 2014) Advisors: Prof. David Collison, Prof. Eric McInnes, Prof. Richard Winpenny Master’s in Chemistry, University of Manchester (2006 – 2010) Honors International Institute for Nanotechnology Outstanding Researcher Award (2017) Activities and Interests My research interests center on the use of extremely high pressure for the synthesis of completely new structures and chemical bonds. More broadly, I am interested in the use of X-ray crystallography as a tool to examine reaction mechanism in solid-state chemistry. I am a frequent user of the HPCAT and GSECARS beamlines at the APS. I collaborate closely with beamline scientists across both sectors and have averaged 8 shifts each run over the last four years. The APS is a world leader in the field of high pressure and is the source of many of the cutting-edge techniques that have since been adopted by other beamlines. This trend of origination is set to continue with the upgrade, which will position the APS at the forefront of synchrotron radiation science. The enormous increase in flux will make it the flagship of a new generation of experiments that allow for crystallographic access to unprecedented ultrafast timescales. -

School of Chemical Engineering and Analytical Sciences Catalytic Research Prof
Faculty of Sciences and Engineering Faculty of Engineering and Physical Sciences School of Chemical Engineering and Analytical Sciences Catalytic Research Prof. Chris Hardacre School of Chemical Engineering and Analytical Science [email protected] Chris Hardacre is Head of the School of Chemical Engineering and Analytical Science and Professor of Chemical Engineering, with research interests in heterogeneous catalysis, in-situ method development and ionic liquids. He has 350+ publications with an H-index of 65 and over 15,000 citations. He is a Member of the Royal Irish Academy, Fellow of the Institute of Chemical Engineering and Fellow of Royal Society of Chemistry. He has a number of awards including the inaugural Andrew Medal for catalysis and has won ~£28M research grant over the past 20 years. We are a world-leading research group working on heterogeneous catalysis and ionic liquids. We have developed a number of state-of-the-art techniques for in- situ monitoring of the systems studied and have strong links with industry. We target applications in energy, bulk, fine and pharmaceutical chemical synthesis as well as environmental protection: •Non-thermal plasma catalysis. ACS Catal., 2015, 5 956; 2014, 4, 666; •Neutron and X-ray scattering studies of catalysts and ionic liquids, Chem. Sci., 2013, 4, 3484; 2013, 4, 1270; 2011, 2, 1594; •Activating gold catalysts. ACS Catal., 2012, 2, 552; Angew. Chem. Int. Ed., 2011, 50, 8912; JACS, 2009, 131, 6973; •Electrochemical reduction of CO2. Angew. Chem. Int. Ed., 2015, 54, 14164.(Hot -
The 17Th International Conference on Molecule-Based Magnets Online
The 17th International Conference on Molecule-based Magnets Online via The University of Manchester 14 - 18 June 2021 Sponsors 2 Knowledge for your next step forward Volume 9 Number 1 7 January 2018 C h e m i c a l Pages 1-268 C h e m i c a l S c i e n c e S c i e n c e rsc.li/chemical-science Open and free, for authors and readers The Royal Society of Chemistry’s flagship journal introduces primary research in all fields to a global readership Editor-in-chief ISSN 2041-6539 EDGE ARTICLE Andrew Cooper University of Liverpool, UK Xinjing Tang et al. Caged circular siRNAs for photomodulation of gene expression in cells and mice Submit your work rsc.li/chemical-science @ChemicalScience Volume 46 Number 10 14 March 2017 D a l t o n Pages 3073-3412 Dalton Transactions T r a n s a c t i o n s An international journal of inorganic chemistry rsc.li/dalton The international journal for high quality, original research in inorganic and organometallic chemistry Fast times to publication mean rapid visibility for your work Editorial Board Chair Russell Morris University of St Andrews, UK ISSN 0306-0012 COMMUNICATION Douglas W. Stephen et al. Submit your work N-Heterocyclic carbene stabilized parent sulfenyl, selenenyl, and tellurenyl cations (XH+, X = S, Se, Te) rsc.li/dalton @DaltonTrans Get journal updates: rsc.li/alerts Registered charity number: 207890 Contents Sponsors ..................................................................................................................................... 2 Contents ..................................................................................................................................... 3 Programme ................................................................................................................................. 4 Prof. Keith Murray: Olivier Kahn Lecture ..................................................................................... 5 Prof. -
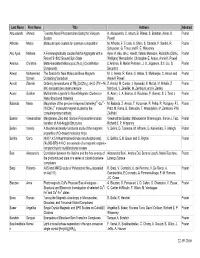
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A. Abouelwafa, C. Anson, B. Pilawa, S. Balaban, Annie. K. Poster System Powell Affronte Marco Molecular spin clusters for quantum computation M. Affronte, F. Troiani, A. Ghirri, S. Carretta, P. Santini, R. Poster Schuecker, G. Timco and R. E. Winpenny Ako Ayuk Manase A Ferromagnetically coupled Mn19 Aggregate with a Ayuk M. Ako, Ian J. Hewitt, Valeriu Mereacre, Rodolphe Clérac, Poster Record S=83/2 Ground Spin State Wolfgang Wernsdorfer, Christopher E. Anson, Annie K. Powell Ambrus Christina Metal-templated Macrocyclic (N3O2) Coordination C.Ambrus, B. Møller Petersen, J. O. Jeppesen, S.X. Liu, S. Poster Compounds Decurtins Anwar Muhammad The Search for New Molecular Base Magnets M. U. Anwar, R. Kania, G. Abbas, S. Mukherjee, C. Anson and Poster Usman Containing Vanadium Annie K Powell Arnold Zdenek Ordering temperatures of TM3[Cr(CN)6]2.nH2O (TM – Ni, Z. Arnold, M. Cieslar, J. Kamarád, S. Maťaš, M. Mihalik, Z. Poster Mn) nanoparticles under pressure Mitróová, V. Zeleňák, M. Zentková, and A. Zentko Aromí Guillem Multidentate Ligands for Novel Magnetic Clusters or G. Aromí, L. A. Barrios, O. Roubeau, P. Gamez, S. J. Teat, J. Poster Nano-Structured Materials Ribas 5+ 2+ Balanda Maria Magnetism of the genuine bi-layered (tetrenH5) -Cu - M. Bałanda, Z. Arnold, T. Korzeniak, R. Pełka, R. Podgajny, F.L. Poster 3- [W(CN)8] molecular magnet studied by the Pratt, M. Rams, B. Sieklucka, T. Wasiutyński, M. Zentkova, P.M. complementary methods Zieliński Baskar Viswanathan Manganese, Zinc and Sodium Polyoxoantimonates: Viswanathan Baskar, Maheswaran Shanmugam, Simon J. -

James P. S. Walsh
James P. S. Walsh [email protected] University of Massachusetts Amherst Department of Chemistry +1 (413) 545–1557 Physical Sciences Building http://jpswalsh.com 690 N Pleasant St [email protected] Amherst, MA 01003 RESEARCH POSITIONS Assistant Professor Sep 2019 – Present University of Massachusetts Amherst, United States Postdoctoral Fellow with Prof Danna Freedman May 2015 – Aug 2019 Northwestern University, United States Postdoctoral Fellow with Dr Jacob Overgaard Mar 2015 – May 2015 Aarhus University, Denmark Research Associate with Dr Alistair Fielding Nov 2014 – Feb 2015 University of Manchester, United Kingdom EDUCATION PhD in Inorganic Chemistry Sep 2010 – Oct 2014 Nanoscience Doctoral Training Centre, University of Manchester, United Kingdom Advisors: Prof David Collison, Prof Eric McInnes, and Prof Richard Winpenny MChem in Chemistry with Forensic Science Sep 2006 – Aug 2010 University of Manchester, United Kingdom HONOURS AND AWARDS COMPRES Postdoc Travel Scholarship (Annual Meeting, Santa Ana Pueblo, New Mexico, USA) Aug 2018 IUCr-HP Early Career Travel Award (IUCr Commission on High-Pressure, Honolulu, Hawaiʻi, USA) Jul 2018 Northwestern Postdoctoral Professional Development Travel Award Dec 2017 International Institute for Nanotechnology Outstanding Researcher Award Sep 2017 COMPRES Postdoc Travel Scholarship (Annual Meeting, Santa Ana Pueblo, New Mexico, USA) Jul 2017 Marie Skłodowska-Curie Masterclass Invited Participant (Aarhus University, Denmark) May 2016 INVITED TALKS Special Seminar (Georgia Institute of Technology, -

Controlled Synthesis of Nanoscopic Metal Cages Jesus̀ Ferrando-Soria, Antonio Fernandez, Eufemio Moreno Pineda, Sarah A
This is an open access article published under a Creative Commons Attribution (CC-BY) License, which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. Communication pubs.acs.org/JACS Controlled Synthesis of Nanoscopic Metal Cages Jesus̀ Ferrando-Soria, Antonio Fernandez, Eufemio Moreno Pineda, Sarah A. Varey, Ralph W. Adams, Iñigo J. Vitorica-Yrezabal, Floriana Tuna, Grigore A. Timco, Christopher A. Muryn, and Richard E. P. Winpenny* School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester M13 9PL, U.K. *S Supporting Information such, they are two-level systems and are candidates as ABSTRACT: Here we show an elegant and general route molecular qubits,14 e.g., they have sufficient phase memory to the assembly of a giant {M12C24} cage from 12 times to allow gate operations before state degradation can palladium ions (M) and 24 heterometallic octanuclear occur,15 and it is also possible to control the interaction 16 coordination cages (C = {Cr7Ni-Py2}). The molecule is 8 between the heterometallic rings. A major question as we nm in size, and the methods for its synthesis and move toward scalable quantum devices is whether sufficient characterization provide a basis for future developments molecular qubits can be incorporated in one assembly while at this scale. retaining the phase memory time; here we show that this is possible in an assembly containing 24 qubits. We first functionalize the inorganic building block to obtain anoscopic objects falling around 10 nm in size are the groups needed to assemble the target nanoscopic cage difficult to make in a monodisperse form, as traditional (Figure 1a) and then carry out a self-assembly reaction (Figure N − approaches to nanoparticle synthesis tend to produce 1b). -

High-Frequency and High-Field EPR/ESR in Tallahassee, FL
2012 epr volume 22 number 2 news A letter zz R PELDOR PELDOR signals The Publication of the International time (ns) EPR (ESR) Society newsepr letter www.epr-newsletter.ethz.ch Officers of the international ePr (esr) society The official publication of the International EPR (ESR) Society is supported by the Society, by corporate and President secretArY other donors, the Zavoisky Physical-Technical Institute of the Russian Academy of Sciences, Kazan, Russian seigo Yamauchi sushil K. Misra Federation, and the Swiss Federal Institute of Technology, Institute of Multidisciplinary Research for Concordia University, Zürich, Switzerland. Advanced Materials (IMRAM), 1455 de Maisonneuve Boulevard West, Tohoku University, Montreal (Quebec), H3G 1M8, Canada Katahira-2-1-1, phone: 514-848-2424 ext. 3278, fax: 514-848-2828 editOr Aobaku, Sendai 980-8577, Japan e-mail: [email protected] Laila V. Mosina phone: 81-22-217-5617, fax: 81-22-217-5616 web: physics.concordia.ca/faculty/misra.php Zavoisky Physical-Technical Institute e-mail: [email protected] Russian Academy of Sciences treAsurer Kazan, Russian Federation Vice Presidents tatyana i. smirnova [email protected] Americas North Carolina State University, AssOciAte editOrs Lawrence Berliner Department of Chemistry, Candice S. Klug Department of Chemistry and Biochemistry, Campus Box 8204, Raleigh, NC 27695-8204, USA Medical College of Wisconsin University of Denver, phone: (919) 513-4375, fax: (919) 513-7353 Milwaukee, WI, USA 2090 E. Iliff Ave, Denver, CO, OR 80208 USA e-mail: [email protected] [email protected] phone: 303-871-7476, fax: 303-871-2254 Hitoshi Ohta e-mail: [email protected] ImmediAte PAst President Molecular Photoscience Research Center, web: www.du.edu/chemistry/Faculty/lberliner.html Jack H.