Part III Chemistry Course Unit Directory
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

N Ew Slet T Er
Electron paramagnetic resonance spectroscopy Now located in the PSI, the EPSRC National EPR Research Facility and Service is run by Profs David Collison and Eric McInnes from the School of Chemistry and has been established with £4.1M fund- ing from EPSRC, and £355K from the University of Manchester. The Facility accommodates several Bruker EPR in- struments, allowing c.w. and pulsed EPR measure- ments at frequencies between 1 (L-band) and 95 GHz (W-band), a Quantum Design magnetometer and an ODESSA instrument (built by Dr Nigel Poolton) The EPR Team from right to left: Prof Eric McInnes, Dr Stephen Sproules, Prof David Collison, Dr Floriana Tuna, that combines optically detected magnetic resonance Dr Brian Tolson, Miss Chloe Stott, Dr Nigel Poolton (absent) (ODMR) with photo-EPR (both 34 GHz; 0-4 T mag- Facilities: Frequency (GHz): 1, 4, 9.5, 24, 34, 94 net). Together these make a unique research base for Frequency Band: L-, S-, X-, K-, Q-, W- studying various types of paramagnetic species and Modes: Parallel and perpendicular at X-band materials. Temperature Range: 4.2 to 300 K all bands, to 500 K at X-band only Researchers across the country are using the Facility on a regular basis. PhD students and academics are For further details see the Facility website: encouraged to visit the Centre and to gain ―hands on‖ http://www.epr.chemistry.manchester.ac.uk/ experience. Newsletter Winter 2011/12 This newsletter consists of a combination of articles, highlighting both recent grant successes and those of a personal nature. Until further notice, items for future newsletters and/or the PSI website should be sent to [email protected]. -

James Walsh Postdoctoral Fellow Department of Chemistry
James Walsh Postdoctoral Fellow Department of Chemistry Northwestern University Evanston, IL 60208 phone: (847) 491-4356 email: [email protected] Current Position Assistant Professor, Department of Chemistry, University of Massachusetts Amherst (Sep 2019) Postdoctoral Fellow, Department of Chemistry, Northwestern University (2015 – Present) Advisors: Prof. Danna Freedman and Prof. Steven Jacobsen Background Postdoctoral Fellow, Aarhus University (2015) Advisor: Dr. Jacob Overgaard Ph.D. in Inorganic Chemistry, University of Manchester (2010 – 2014) Advisors: Prof. David Collison, Prof. Eric McInnes, Prof. Richard Winpenny Master’s in Chemistry, University of Manchester (2006 – 2010) Honors International Institute for Nanotechnology Outstanding Researcher Award (2017) Activities and Interests My research interests center on the use of extremely high pressure for the synthesis of completely new structures and chemical bonds. More broadly, I am interested in the use of X-ray crystallography as a tool to examine reaction mechanism in solid-state chemistry. I am a frequent user of the HPCAT and GSECARS beamlines at the APS. I collaborate closely with beamline scientists across both sectors and have averaged 8 shifts each run over the last four years. The APS is a world leader in the field of high pressure and is the source of many of the cutting-edge techniques that have since been adopted by other beamlines. This trend of origination is set to continue with the upgrade, which will position the APS at the forefront of synchrotron radiation science. The enormous increase in flux will make it the flagship of a new generation of experiments that allow for crystallographic access to unprecedented ultrafast timescales. -
The 17Th International Conference on Molecule-Based Magnets Online
The 17th International Conference on Molecule-based Magnets Online via The University of Manchester 14 - 18 June 2021 Sponsors 2 Knowledge for your next step forward Volume 9 Number 1 7 January 2018 C h e m i c a l Pages 1-268 C h e m i c a l S c i e n c e S c i e n c e rsc.li/chemical-science Open and free, for authors and readers The Royal Society of Chemistry’s flagship journal introduces primary research in all fields to a global readership Editor-in-chief ISSN 2041-6539 EDGE ARTICLE Andrew Cooper University of Liverpool, UK Xinjing Tang et al. Caged circular siRNAs for photomodulation of gene expression in cells and mice Submit your work rsc.li/chemical-science @ChemicalScience Volume 46 Number 10 14 March 2017 D a l t o n Pages 3073-3412 Dalton Transactions T r a n s a c t i o n s An international journal of inorganic chemistry rsc.li/dalton The international journal for high quality, original research in inorganic and organometallic chemistry Fast times to publication mean rapid visibility for your work Editorial Board Chair Russell Morris University of St Andrews, UK ISSN 0306-0012 COMMUNICATION Douglas W. Stephen et al. Submit your work N-Heterocyclic carbene stabilized parent sulfenyl, selenenyl, and tellurenyl cations (XH+, X = S, Se, Te) rsc.li/dalton @DaltonTrans Get journal updates: rsc.li/alerts Registered charity number: 207890 Contents Sponsors ..................................................................................................................................... 2 Contents ..................................................................................................................................... 3 Programme ................................................................................................................................. 4 Prof. Keith Murray: Olivier Kahn Lecture ..................................................................................... 5 Prof. -
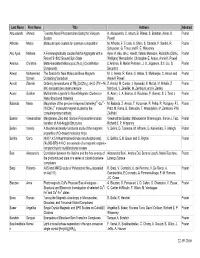
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A. Abouelwafa, C. Anson, B. Pilawa, S. Balaban, Annie. K. Poster System Powell Affronte Marco Molecular spin clusters for quantum computation M. Affronte, F. Troiani, A. Ghirri, S. Carretta, P. Santini, R. Poster Schuecker, G. Timco and R. E. Winpenny Ako Ayuk Manase A Ferromagnetically coupled Mn19 Aggregate with a Ayuk M. Ako, Ian J. Hewitt, Valeriu Mereacre, Rodolphe Clérac, Poster Record S=83/2 Ground Spin State Wolfgang Wernsdorfer, Christopher E. Anson, Annie K. Powell Ambrus Christina Metal-templated Macrocyclic (N3O2) Coordination C.Ambrus, B. Møller Petersen, J. O. Jeppesen, S.X. Liu, S. Poster Compounds Decurtins Anwar Muhammad The Search for New Molecular Base Magnets M. U. Anwar, R. Kania, G. Abbas, S. Mukherjee, C. Anson and Poster Usman Containing Vanadium Annie K Powell Arnold Zdenek Ordering temperatures of TM3[Cr(CN)6]2.nH2O (TM – Ni, Z. Arnold, M. Cieslar, J. Kamarád, S. Maťaš, M. Mihalik, Z. Poster Mn) nanoparticles under pressure Mitróová, V. Zeleňák, M. Zentková, and A. Zentko Aromí Guillem Multidentate Ligands for Novel Magnetic Clusters or G. Aromí, L. A. Barrios, O. Roubeau, P. Gamez, S. J. Teat, J. Poster Nano-Structured Materials Ribas 5+ 2+ Balanda Maria Magnetism of the genuine bi-layered (tetrenH5) -Cu - M. Bałanda, Z. Arnold, T. Korzeniak, R. Pełka, R. Podgajny, F.L. Poster 3- [W(CN)8] molecular magnet studied by the Pratt, M. Rams, B. Sieklucka, T. Wasiutyński, M. Zentkova, P.M. complementary methods Zieliński Baskar Viswanathan Manganese, Zinc and Sodium Polyoxoantimonates: Viswanathan Baskar, Maheswaran Shanmugam, Simon J. -

James P. S. Walsh
James P. S. Walsh [email protected] University of Massachusetts Amherst Department of Chemistry +1 (413) 545–1557 Physical Sciences Building http://jpswalsh.com 690 N Pleasant St [email protected] Amherst, MA 01003 RESEARCH POSITIONS Assistant Professor Sep 2019 – Present University of Massachusetts Amherst, United States Postdoctoral Fellow with Prof Danna Freedman May 2015 – Aug 2019 Northwestern University, United States Postdoctoral Fellow with Dr Jacob Overgaard Mar 2015 – May 2015 Aarhus University, Denmark Research Associate with Dr Alistair Fielding Nov 2014 – Feb 2015 University of Manchester, United Kingdom EDUCATION PhD in Inorganic Chemistry Sep 2010 – Oct 2014 Nanoscience Doctoral Training Centre, University of Manchester, United Kingdom Advisors: Prof David Collison, Prof Eric McInnes, and Prof Richard Winpenny MChem in Chemistry with Forensic Science Sep 2006 – Aug 2010 University of Manchester, United Kingdom HONOURS AND AWARDS COMPRES Postdoc Travel Scholarship (Annual Meeting, Santa Ana Pueblo, New Mexico, USA) Aug 2018 IUCr-HP Early Career Travel Award (IUCr Commission on High-Pressure, Honolulu, Hawaiʻi, USA) Jul 2018 Northwestern Postdoctoral Professional Development Travel Award Dec 2017 International Institute for Nanotechnology Outstanding Researcher Award Sep 2017 COMPRES Postdoc Travel Scholarship (Annual Meeting, Santa Ana Pueblo, New Mexico, USA) Jul 2017 Marie Skłodowska-Curie Masterclass Invited Participant (Aarhus University, Denmark) May 2016 INVITED TALKS Special Seminar (Georgia Institute of Technology, -

PSI Newsletter
Summer 2012 PSI Newsletter Inside this issue: Frogs and Physics 1 Frogs and Physics Dr Mark Dickinson and Andrew Gray, Curator of 1 Chemical Society review Herpetology at the Manchester Museum have been ex- accepted ploring the world of frogs with a class of Year 12 Physics students at the Manchester Museum. 2 A Message from the Director The day started with an introduction into the use of col- 3 Photon 12 our in the animal kingdom. The students were also intro- A student meets Prince 3 Branchpoint expansion in a fully duced to a “glass frog” (where you can see its internal Charming (an extremely rare complementary three-way organs) and a “splendid tree frog”, one of only about a splendid tree frog) DNA junction hundred left in the world. 3 Three dimensional optical im- Andrew explained how frogs were dying out in the rain forests of Central and South aging of actinide ions using two America as a result of a fungus. His research, which combines field studies with captive photon spectroscopy observations, focuses mainly on investigating the biology of the rare treefrog species. All the studies conducted are completely non-invasive and are aimed at gaining a fuller un- Chemical engineering of mo- 4 derstanding of the species concerned, so that the knowledge can be used to help con- lecular qubits - a joint publica- tion between MIB and PSI serve them. Physicist Dr Mark Dickinson from the Photon Science Institute then explained the phys- 4 Louise Natrajan visits the INE ics behind the research, including the use of visible and infrared techniques to analyse the 5 Detecting free radicals in the frog’s skin, and a master class in infrared techniques.