Method for Highly Sensitive Determination of NADH Using NADH Kinase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

METACYC ID Description A0AR23 GO:0004842 (Ubiquitin-Protein Ligase
Electronic Supplementary Material (ESI) for Integrative Biology This journal is © The Royal Society of Chemistry 2012 Heat Stress Responsive Zostera marina Genes, Southern Population (α=0. -

Ribonucleotides Incorporated by the Yeast Mitochondrial DNA Polymerase Are Not Repaired
Ribonucleotides incorporated by the yeast mitochondrial DNA polymerase are not repaired Paulina H. Wanrooija,1, Martin K. M. Engqvistb,c,2, Josefin M. E. Forslunda,2, Clara Navarreteb, Anna Karin Nilssona, Juhan Sedmand, Sjoerd Wanrooija, Anders R. Clausenb, and Andrei Chabesa,e,1 aDepartment of Medical Biochemistry and Biophysics, Umeå University, SE-901 87 Umeå, Sweden; bInstitute of Biomedicine, University of Gothenburg, SE-405 30 Gothenburg, Sweden; cDepartment of Biology and Biological Engineering, Chalmers University of Technology, SE-412 96 Gothenburg, Sweden; dDepartment of Biochemistry, Institute of Molecular and Cell Biology, University of Tartu, Tartu 51010, Estonia; and eLaboratory for Molecular Infection Medicine Sweden, Umeå University, SE-901 87 Umeå, Sweden Edited by Philip C. Hanawalt, Stanford University, Stanford, CA, and approved October 17, 2017 (received for review July 25, 2017) Incorporation of ribonucleotides into DNA during genome replica- Mec1/Rad53 genome integrity checkpoint regulates yeast RNR tion is a significant source of genomic instability. The frequency activity through several different mechanisms (14). of ribonucleotides in DNA is determined by deoxyribonucleoside The incorporation of ribonucleotides (rNMPs) into the genome triphosphate/ribonucleoside triphosphate (dNTP/rNTP) ratios, by the during DNA replication has become recognized as a significant ability of DNA polymerases to discriminate against ribonucleotides, source of genomic instability. Given that the physiological con- and by the capacity of repair mechanisms to remove incorporated centrations of ribonucleoside triphosphates (rNTPs), the building ribonucleotides. To simultaneously compare how the nuclear and blocks of RNA, are one to two orders-of-magnitude higher than mitochondrial genomes incorporate and remove ribonucleotides, those of dNTPs, rNMPs are frequently incorporated into DNA we challenged these processes by changing the balance of cellular during replication (15, 16). -

Table S1. List of Oligonucleotide Primers Used
Table S1. List of oligonucleotide primers used. Cla4 LF-5' GTAGGATCCGCTCTGTCAAGCCTCCGACC M629Arev CCTCCCTCCATGTACTCcgcGATGACCCAgAGCTCGTTG M629Afwd CAACGAGCTcTGGGTCATCgcgGAGTACATGGAGGGAGG LF-3' GTAGGCCATCTAGGCCGCAATCTCGTCAAGTAAAGTCG RF-5' GTAGGCCTGAGTGGCCCGAGATTGCAACGTGTAACC RF-3' GTAGGATCCCGTACGCTGCGATCGCTTGC Ukc1 LF-5' GCAATATTATGTCTACTTTGAGCG M398Arev CCGCCGGGCAAgAAtTCcgcGAGAAGGTACAGATACGc M398Afwd gCGTATCTGTACCTTCTCgcgGAaTTcTTGCCCGGCGG LF-3' GAGGCCATCTAGGCCATTTACGATGGCAGACAAAGG RF-5' GTGGCCTGAGTGGCCATTGGTTTGGGCGAATGGC RF-3' GCAATATTCGTACGTCAACAGCGCG Nrc2 LF-5' GCAATATTTCGAAAAGGGTCGTTCC M454Grev GCCACCCATGCAGTAcTCgccGCAGAGGTAGAGGTAATC M454Gfwd GATTACCTCTACCTCTGCggcGAgTACTGCATGGGTGGC LF-3' GAGGCCATCTAGGCCGACGAGTGAAGCTTTCGAGCG RF-5' GAGGCCTGAGTGGCCTAAGCATCTTGGCTTCTGC RF-3' GCAATATTCGGTCAACGCTTTTCAGATACC Ipl1 LF-5' GTCAATATTCTACTTTGTGAAGACGCTGC M629Arev GCTCCCCACGACCAGCgAATTCGATagcGAGGAAGACTCGGCCCTCATC M629Afwd GATGAGGGCCGAGTCTTCCTCgctATCGAATTcGCTGGTCGTGGGGAGC LF-3' TGAGGCCATCTAGGCCGGTGCCTTAGATTCCGTATAGC RF-5' CATGGCCTGAGTGGCCGATTCTTCTTCTGTCATCGAC RF-3' GACAATATTGCTGACCTTGTCTACTTGG Ire1 LF-5' GCAATATTAAAGCACAACTCAACGC D1014Arev CCGTAGCCAAGCACCTCGgCCGAtATcGTGAGCGAAG D1014Afwd CTTCGCTCACgATaTCGGcCGAGGTGCTTGGCTACGG LF-3' GAGGCCATCTAGGCCAACTGGGCAAAGGAGATGGA RF-5' GAGGCCTGAGTGGCCGTGCGCCTGTGTATCTCTTTG RF-3' GCAATATTGGCCATCTGAGGGCTGAC Kin28 LF-5' GACAATATTCATCTTTCACCCTTCCAAAG L94Arev TGATGAGTGCTTCTAGATTGGTGTCggcGAAcTCgAGCACCAGGTTG L94Afwd CAACCTGGTGCTcGAgTTCgccGACACCAATCTAGAAGCACTCATCA LF-3' TGAGGCCATCTAGGCCCACAGAGATCCGCTTTAATGC RF-5' CATGGCCTGAGTGGCCAGGGCTAGTACGACCTCG -

Mitochondrial Uncoupling and the Reprograming of Intermediary Metabolism in Leukemia Cells
REVIEW ARTICLE published: 02 April 2013 doi: 10.3389/fonc.2013.00067 Mitochondrial uncoupling and the reprograming of intermediary metabolism in leukemia cells Juliana Vélez 1, Numsen Hail Jr.2,3, Marina Konopleva2,3, Zhihong Zeng 2,3, Kensuke Kojima2,3, Ismael Samudio1* and Michael Andreeff 2,3* 1 Grupo de Terapia Celular y Molecular Laboratorio de Bioquimica, Pontificia Universidad Javeriana, Bogotá, Colombia 2 Section of Molecular Hematology and Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX, USA 3 Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, USA Edited by: Nearly 60 years ago Otto Warburg proposed, in a seminal publication, that an irreparable Jozsef Dudas, Medical University defect in the oxidative capacity of normal cells supported the switch to glycolysis for energy Innsbru, Austria generation and the appearance of the malignant phenotype (Warburg, 1956). Curiously, Reviewed by: Daniel Christian Hoessli, International this phenotype was also observed by Warburg in embryonic tissues, and recent research Center for Chemical and Biological demonstrated that normal stem cells may indeed rely on aerobic glycolysis – fermenting Sciences, Switzerland pyruvate to lactate in the presence of ample oxygen – rather than on the complete oxidation Jozsef Dudas, Medical University of pyruvate in the Krebs cycle – to generate cellular energy (Folmes et al., 2012). However, Innsbruck, Austria it remains to be determined whether this phenotype is causative for neoplastic develop- *Correspondence: Ismael Samudio, Laboratorio de ment, or rather the result of malignant transformation. In addition, in light of mounting Bioquimica #301, Edificio Jesús evidence demonstrating that cancer cells can carry out electron transport and oxidative Emilio Ramírez, Pontificia Universidad phosphorylation, although in some cases predominantly using electrons from non-glucose Javeriana, Cra 7 No. -

Genome-Wide Investigation of Cellular Functions for Trna Nucleus
Genome-wide Investigation of Cellular Functions for tRNA Nucleus- Cytoplasm Trafficking in the Yeast Saccharomyces cerevisiae DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Hui-Yi Chu Graduate Program in Molecular, Cellular and Developmental Biology The Ohio State University 2012 Dissertation Committee: Anita K. Hopper, Advisor Stephen Osmani Kurt Fredrick Jane Jackman Copyright by Hui-Yi Chu 2012 Abstract In eukaryotic cells tRNAs are transcribed in the nucleus and exported to the cytoplasm for their essential role in protein synthesis. This export event was thought to be unidirectional. Surprisingly, several lines of evidence showed that mature cytoplasmic tRNAs shuttle between nucleus and cytoplasm and their distribution is nutrient-dependent. This newly discovered tRNA retrograde process is conserved from yeast to vertebrates. Although how exactly the tRNA nuclear-cytoplasmic trafficking is regulated is still under investigation, previous studies identified several transporters involved in tRNA subcellular dynamics. At least three members of the β-importin family function in tRNA nuclear-cytoplasmic intracellular movement: (1) Los1 functions in both the tRNA primary export and re-export processes; (2) Mtr10, directly or indirectly, is responsible for the constitutive retrograde import of cytoplasmic tRNA to the nucleus; (3) Msn5 functions solely in the re-export process. In this thesis I focus on the physiological role(s) of the tRNA nuclear retrograde pathway. One possibility is that nuclear accumulation of cytoplasmic tRNA serves to modulate translation of particular transcripts. To test this hypothesis, I compared expression profiles from non-translating mRNAs and polyribosome-bound translating mRNAs collected from msn5Δ and mtr10Δ mutants and wild-type cells, in fed or acute amino acid starvation conditions. -

Table S3 Abbreviations Variable Name in Program Definition Or Full
Table S3 Abbreviations Variable name in program definition or full name tot_Glc_B total input glucose t_delay_Glc_B time delay of glucose release tot_TG_B total input TG t_delay_TG_B time dealy of TG release k_inssyn_B insulin base rate constant Vmax_inssyn_Glc_B insulin synthesis rate constant for plasma glucose Km_inssyn_Glc_B Michaelis constant for insulin synthesis n_inssyn_Glc_B Hill coefficient for insulin synthesis k_inssyn_FFA_B insulin synthesis rate constant for plasma FFA k_insdeg_Ins_B insulin degradation rate constant k_choluse_Chol_B cholesterol utilization rate constant in blood alpha_base_L insulin base factor in liver alpha_band_L insulin allowable width in liver Km_Ins_B_L Michaelis constant for insulin in liver n_Ins_B_L Hill coefficient for insulin in liver Vdif_glut2_Glc_B_L rate constant of glucose transporter type 2 in liver Kdif_glut2_Glc_B_L dissociation constant of glucose transporter type 2 for Glc_B in liver Kdif_glut2_Glc_L dissociation constant of glucose transporter type 2 in liver Vmax_hk_Glc_L reaction rate constant of hexiokinase for Glc in liver Km_hk_Glc_L Michaelis constant of hexiokinase for Glc in liver Ki_hk_G6p_L inhibition constant of hexiokinase for Glc in liver Km_hk_Atp_L Michaelis constant of hexiokinase for Atp in liver Ki_hk_Atp_L inhibition constant of hexiokinase for Atp in liver Vmax_ppp_G6p_L reaction rate constant of pentose phosphate pathway for G6p in liver Km_ppp_G6p_L Michaelis constant of pentose phosphate pathway for G6p in liver Km_ppp_Nadp_L Michaelis constant of pentose phosphate -

Over-Expression of NAD Kinase in Corynebacterium Crenatum and Its Impact on L-Arginine Biosynthesis
Ezzeldi & Nahhas Tropical Journal of Pharmaceutical Research December 2012; 11 (6): 909-916 © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v11i6.6 Research Article Over-expression of NAD kinase in Corynebacterium crenatum and its Impact on L-Arginine Biosynthesis Muhammad Masfiqur Rahman1, Zhao Qin Qin1, Wenfang Dou1, Rao Zhiming1 and Zhenghong Xu1,2* 1Laboratory of Pharmaceutical Engineering, School of Medicine and Pharmaceutics, 2The Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, P R China Abstract Purpose: To improve the biosynthesis of L-arginine by overexpressing homologous NAD kinase (ppnk) in Corynebacterium crenatum SYPA5-5 and to study its impact in presence of high (HOS) and low oxygen supply (LOS). Methods: A recombinant plasmid (pJC1-tac-ppnK) harboring homologous NAD kinase (ppnk) was constructed in a shuttle vector pJC1 and transferred in L-arginine producing strain Corynebacterium crenatum SYPA5-5. Furthermore, fermentation was performed by shake flask method with consecutive determination of cell growth and glucose concentration. NAD+ kinase activity was studied by stop method and NADP(H) concentrations were determined by spectrophotometric enzymatic cycling method. To check the biosynthesis of amino acids, HPLC method was used to determine extracellular amino acid concentrations. Results: In HOS condition, NAD+ kinase activity increased by 116 %, while intracellular concentrations of NADP+ and NADPH increased by 7.3 and 36.8 %, respectively. Whereas, in LOS condition , NAD+ kinase activity increased 49 % , with intracellular 14.67 and 15 % increases in NADP+ and NADPH respectively. -

Source: the Arabidopsis Information Resource (TAIR);
Table S1 List of targeted loci and information about their function in Arabidopsis thaliana (source: The Arabidopsis Information Resource (TAIR); https://www.arabidopsis.org/tools/bulk/genes/index.jsp). Locus Gene Model Gene Model Description Gene Model Primary Gene Symbol All Gene Symbols Identifier Name Type AT1G78800 AT1G78800.1 UDP-Glycosyltransferase superfamily protein_coding protein;(source:Araport11) AT5G06830 AT5G06830.1 hypothetical protein;(source:Araport11) protein_coding AT2G31740 AT2G31740.1 S-adenosyl-L-methionine-dependent methyltransferases protein_coding superfamily protein;(source:Araport11) AT5G11960 AT5G11960.1 magnesium transporter, putative protein_coding (DUF803);(source:Araport11) AT4G00560 AT4G00560.4 NAD(P)-binding Rossmann-fold superfamily protein_coding protein;(source:Araport11) AT1G80510 AT1G80510.1 Encodes a close relative of the amino acid transporter ANT1 protein_coding (AT3G11900). AT2G21250 AT2G21250.1 NAD(P)-linked oxidoreductase superfamily protein_coding protein;(source:Araport11) AT5G04420 AT5G04420.1 Galactose oxidase/kelch repeat superfamily protein_coding protein;(source:Araport11) AT4G34910 AT4G34910.1 P-loop containing nucleoside triphosphate hydrolases protein_coding superfamily protein;(source:Araport11) AT5G66120 AT5G66120.2 3-dehydroquinate synthase;(source:Araport11) protein_coding AT1G45110 AT1G45110.1 Tetrapyrrole (Corrin/Porphyrin) protein_coding Methylase;(source:Araport11) AT1G67420 AT1G67420.2 Zn-dependent exopeptidases superfamily protein_coding protein;(source:Araport11) AT3G62370 -

Detection of Biological Material Nachweis Von Biologischem Materal Detection De Substances Biologiques
Europaisches Patentamt (19) European Patent Office Office europeenpeen des brevets EP 0 695 363 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) intci.6: C12Q 1/00, C12Q 1/48, of the grant of the patent: C12Q 1/54 17.09.1997 Bulletin 1997/38 (86) International application number: (21) Application number: 94912610.6 PCT/GB94/00783 Date of 14.04.1994 (22) filing: (87) International publication number: WO 94/25619 (10.11.1994 Gazette 1994/25) (54) DETECTION OF BIOLOGICAL MATERIAL NACHWEIS VON BIOLOGISCHEM MATERAL DETECTION DE SUBSTANCES BIOLOGIQUES (84) Designated Contracting States: (74) Representative: Perry, Robert Edward AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL GILL JENNINGS & EVERY PT SE Broadgate House 7 Eldon Street (30) Priority: 23.04.1993 GB 9308411 London EC2M 7LH (GB) (43) Date of publication of application: (56) References cited: 07.02.1996 Bulletin 1996/06 EP-A- 0 060 123 EP-A- 0 102 504 EP-A- 0 193 895 EP-A- 0 327 952 (73) Proprietor: CELSIS INTERNATIONAL pic GB-A- 2 055 200 Cambridge CB4 4FX (GB) PATENT ABSTRACTS OF JAPAN vol. 1 3, no. 205 (72) Inventors: (C-595) 1 5 May 1 989 & JP, A,01 023 900 (SANKYO • FOOTE, Nicholas, Peter, Martin CO LTD) 26 January 1989 Cambridge CB1 6UF(GB) BIOCHEMICAL SOCIETY TRANSACTIONS, • GRANT, Peter, Leonard vol.19, no.160S, April 1991, LONDON R. S. Cambridge PE17 3UE (GB) CHITTOCK ET AL. 'LIGHT AMPLIFICATION BY A COUPLED BIOLOGICAL SYSTEM: ATP, FIREFLY LUCIFERASE AND RECYCLING OF ATP.' cited in the application DO CO CO CO lO O) CO Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice the Patent Office of the Notice of shall be filed in o to European opposition to European patent granted. -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -
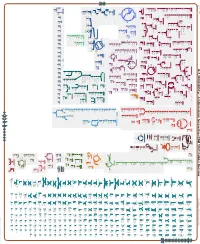
Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_000023265Cyc: Acidimicrobium ferrooxidans DSM 10331 Cellular Overview Connections between pathways are omitted for legibility. Anamika Kothari Macromolecule Modification Fatty Acid and Amine and C1 Compound Lipid Degradation Degradation/ Polyamine Utilization and Utilization/ UDP-N-acetyl- undecaprenyl- sn-glycero-3- apo-[propionyl- a peptidoglycan L-alanyl-γ-D- Biosynthesis tRNA processing Assimilation β Assimilation - Other cob(II)yrinate a,c- Cofactor, Carrier, and Vitamin Biosynthesis oleate -oxidation α-D-glucosamine diphospho-N- 2-methylbutanoate phospho-N- CoA:carbon- 2-oxobutanoate pyruvate dimer (meso- glutamyl-L-lysyl- a protein cob(II)yrinate a,c-diamide flavin biosynthesis I (bacteria and plants) adenosylcobinamide-GDP mycothiol biosynthesis superpathway of thiamine diphosphate biosynthesis II benzimidazolyl adenosylcobamide Fatty Acid and Lipid Biosynthesis ATP roseoflavin biotin RS01180 biotin diamide biosynthesis I putrescine formate acrylonitrile acetylmuramoyl- (arachidonoyl) dioxide ligase -

Class 2 . Transferases Ix Ec 2. 7. 1. 38 - 2
CLASS 2 . TRANSFERASES IX EC 2. 7. 1. 38 - 2. 7. 1. 112 1ST EDITION PDF, EPUB, EBOOK Dietmar Schomburg | 9783662500774 | | | | | Class 2 . Transferases IX Ec 2. 7. 1. 38 - 2. 7. 1. 112 1st edition PDF Book Read this book on SpringerLink. Glucosephosphate phosphodismutase Pages Glucosephosphate phosphodismutase. Shikimate kinase. Protamine kinase. Propionate kinase Pages Offers concise and complete description of about 5, enzymes sufficiently well characterized as well as their application in analytical, synthetic and biotechnology processes, in food industry, and for medicinal treatments. Anthocyanidin 3-O- glucosyltransferase Pages Recommended for you. It seems that you're in Germany. Hydroxylysine kinase. Diacylglycerol kinase Pages Phosphoglucan, water dikinase Pages We have a dedicated site for Germany. The total amount of material contained in the Handbook has more than doubled so that the complete second edition consists of 39 volumes as well as a Synonym Index. Alkylglycerol kinase Pages Buy Softcover. New datafields are created: application and engineering for the properties of enzymes where the sequence has been changed. About this book Springer Handbook of Enzymes provides data on enzymes sufficiently well characterized. New datafields are created: application and engineering for the properties of enzymes where the sequence has been changed. Springer Handbook of Enzymes provides data on enzymes sufficiently well characterized. It seems that you're in Germany. Show all. Diphosphate-serine phosphotransferase Pages Buy eBook. Show all. Springer Handbook of Enzymes Free Preview. D -Ribulokinase. N2- 2-Carboxyethyl arginine synthase Pages Phosphoenolpyruvate-fructose phosphotransferase Pages This new, second edition reflects considerable progress in enzymology: many enzymes are newly classified or reclassified.