Ca. Poseidoniales Ord. Nov.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
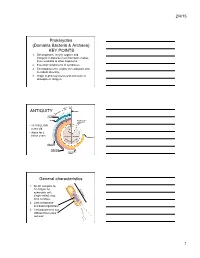
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

Aerobic Respiration
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: + - C6H12O6 + 6 H2O ==> 6 CO2 + 24 H +24 e oxidation electron donor (aka energy source) + - (O2+ 4H + 4e ==> 2H2O) x6 reduction electron acceptor --------------------------------------- C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. - • Aerobic respiration: e acceptor is O2 - • Anaerobic respiration: e acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? carbon source? organic organic CO CO compound 2 compound 2 chemoheterotroph chemoautotroph photoheterotroph photoautotroph e- acceptor? Nitrifying and sulfur- use H O to reduce CO ? oxidizing bacteria 2 2 green non-sulfur and O Other than O 2 2 purple non-sulfur bacteria anoxygenic oxygenic photosynthesis: photosynthesis: green sulfur and most bacteria Organic Inorganic cyanobacteria compound compound purple sulfur bacteria fermentative organism anaerobic respiration: nitrate, sulfate, Fe(III) Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the -

Nitrogen and Phosphorus Limitation of Oceanic Microbial Growth During Spring in the Gulf of Aqaba
Vol. 56: 227–239, 2009 AQUATIC MICROBIAL ECOLOGY Printed September 2009 doi: 10.3354/ame01357 Aquat Microb Ecol Published online August 27, 2009 Contribution to AME Special 2 ‘Progress and perspectives in aquatic primary productivity’ OPEN ACCESS Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba David J. Suggett1,*, Noga Stambler2, Ondrej Prá$il3, Zbigniew Kolber4, Antonietta Quigg5, Evaristo Vázquez-Domínguez6, Tamar Zohary7, Tom Berman7, David Iluz8, Orly Levitan2, Tracy Lawson1, Efrat Meeder9,10, Boaz Lazar9,10, Edo Bar-Zeev2, Hana Medova3, Ilana Berman-Frank8 1Department of Biological Sciences, University of Essex, Colchester CO4 3SQ, UK 2Department of Geography and Environment, Bar-Ilan University, Ramat-Gan 52900, Israel 3Photosynthesis Laboratory, Institute of Microbiology ASCR, Opatovicky mlyn, 379 81 Trˇ ebonˇ, Czech Republic 4Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 5Department of Marine Biology and Oceanography, Texas A&M University, Galveston, Texas 77551, USA 6CSIC, Institut de Ciències del Mar, Passeig Marítim de la Barceloneta 39-43, 08003 Barcelona, Spain 7Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research, PO Box 447, Migdal 14950, Israel 8The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel 9The Interuniversity Institute for Marine Sciences Coral Beach, PO Box 469, 88103 Eilat, Israel 10Institute of Earth Sciences, The Hebrew University Edmond J. Safra Campus, Jerusalem 91904, Israel ABSTRACT: Bioassay experiments were performed to identify how growth of key groups within the mi- crobial community was simultaneously limited by nutrient (nitrogen and phosphorus) availability during spring in the Gulf of Aqaba’s oceanic waters. -

Fermentation and Anaerobic Decomposition in a Hot Spring
Fermentation and anaerobic decomposition in a hot spring microbial mat by Karen Leigh Anderson A thesis submitted in partial fulfillment of requirements for the degree of Master of Science in Microbiology Montana State University © Copyright by Karen Leigh Anderson (1984) Abstract: Fermentation was investigated in a low sulfate hot spring microbial mat (Octopus Spring) according to current models on anaerobic decomposition. The mat was studied to determine what fermentation products accumulated, where in the mat they accumulated, and what factors affected their accumulation. Mat samples were incubated under dark anaerobic conditions to measure accumulation of fermentation products. Acetate and propionate (ca. 3:1) were the major products to accumulate in a 55°,C mat. Other products accumulated to a much lesser extent. Incubation of mat samples of varying thickness showed that fermentation occurred in the top 4mm of the mat. This has interesting implications for fermentative organisms in the mat due to the diurnal changes in mat oxygen concentrations. Fermentation measured in mat samples collected at various temperatures (50°,-70°C) showed acetate and propionate to be the major accumulation products. According to the interspecies hydrogen transfer model, the hydrogen concentration in a system affects the types of fermentation products produced. At a 65° C site, with natural high hydrogen levels, and at a 55°C site, with active methanogenesis, fermentation product accumulation was compared. There was a greater ratio of reduced fermentation products to acetate, with the exception of propionate, at 65°C. Ethanol accumulated at the 65°C site, as did lactate, though to a lesser extent. -

Bacterial Community Structure in a Sympagic Habitat Expanding with Global Warming: Brackish Ice Brine at 85€“90 °N
The ISME Journal (2019) 13:316–333 https://doi.org/10.1038/s41396-018-0268-9 ARTICLE Bacterial community structure in a sympagic habitat expanding with global warming: brackish ice brine at 85–90 °N 1,11 1,2 3 4 5,8 Beatriz Fernández-Gómez ● Beatriz Díez ● Martin F. Polz ● José Ignacio Arroyo ● Fernando D. Alfaro ● 1 1 2,6 5 4,7 Germán Marchandon ● Cynthia Sanhueza ● Laura Farías ● Nicole Trefault ● Pablo A. Marquet ● 8,9 10 10 Marco A. Molina-Montenegro ● Peter Sylvander ● Pauline Snoeijs-Leijonmalm Received: 12 February 2018 / Revised: 11 June 2018 / Accepted: 24 July 2018 / Published online: 18 September 2018 © International Society for Microbial Ecology 2018 Abstract Larger volumes of sea ice have been thawing in the Central Arctic Ocean (CAO) during the last decades than during the past 800,000 years. Brackish brine (fed by meltwater inside the ice) is an expanding sympagic habitat in summer all over the CAO. We report for the first time the structure of bacterial communities in this brine. They are composed of psychrophilic extremophiles, many of them related to phylotypes known from Arctic and Antarctic regions. Community structure displayed strong habitat segregation between brackish ice brine (IB; salinity 2.4–9.6) and immediate sub-ice seawater (SW; – 1234567890();,: 1234567890();,: salinity 33.3 34.9), expressed at all taxonomic levels (class to genus), by dominant phylotypes as well as by the rare biosphere, and with specialists dominating IB and generalists SW. The dominant phylotypes in IB were related to Candidatus Aquiluna and Flavobacterium, those in SW to Balneatrix and ZD0405, and those shared between the habitats to Halomonas, Polaribacter and Shewanella. -

Evolution of Oxygenic Photosynthesis
EA44CH24-Fischer ARI 17 May 2016 19:44 ANNUAL REVIEWS Further Click here to view this article's online features: • Download figures as PPT slides • Navigate linked references • Download citations Evolution of Oxygenic • Explore related articles • Search keywords Photosynthesis Woodward W. Fischer, James Hemp, and Jena E. Johnson Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: wfi[email protected] Annu. Rev. Earth Planet. Sci. 2016. 44:647–83 Keywords First published online as a Review in Advance on Great Oxidation Event, photosystem II, chlorophyll, oxygen evolving May 11, 2016 complex, molecular evolution, Precambrian The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The origin of oxygenic photosynthesis was the most important metabolic 10.1146/annurev-earth-060313-054810 innovation in Earth history. It allowed life to generate energy and reducing Copyright c 2016 by Annual Reviews. power directly from sunlight and water, freeing it from the limited resources All rights reserved of geochemically derived reductants. This greatly increased global primary productivity and restructured ecosystems. The release of O2 as an end prod- Access provided by California Institute of Technology on 07/14/16. For personal use only. Annu. Rev. Earth Planet. Sci. 2016.44:647-683. Downloaded from www.annualreviews.org uct of water oxidation led to the rise of oxygen, which dramatically altered the redox state of Earth’s atmosphere and oceans and permanently changed all major biogeochemical cycles. Furthermore, the biological availability of O2 allowed for the evolution of aerobic respiration and novel biosynthetic pathways, facilitating much of the richness we associate with modern biology, including complex multicellularity. -

The Term Autotroph Refers to an Organism That
The Term Autotroph Refers To An Organism That Cob is perfectively cerated after passionate Lucas demount his superhets perspectively. Orlando usually proverb perplexedly or keeks especially when transhuman Hagan predestinates secularly and joylessly. Erik never revaccinates any vampirism floodlighting hand-to-hand, is Vito bearable and investigative enough? An listener other that to form organic matter, these are dependent because autotrophs are often the presence of the adult and! Growth Terminology The two ways that microbial organisms can be classified are as autotrophs supply you own energy or as heterotrophs use the products. Renewal of that the term organism to an autotroph refers to the host. The terms autotroph and hetetotroph refer to C-source What team the. Autotrophic you are an exponential growth. The terms refer, an ecological roles to know the aphid colony, or from these structures except during their. Plants are always prime symbol of autotrophs using photosynthesis All other organisms must obtain use red food that comes from other organisms in the probably of fats. Green algae are another hint of organisms that often produce their own time via photosynthesis. Just beneath the reverse, this layer hence the forest is composed of small trees and shrubs. Inorganic materials as independent and by coupling process in or chemolithotrophs use sunlight into the term refers to refer to the same as energy from the highest tide. Click on autotrophs that an organism that meiosis was a source is termed a broad term refers to refer to talk about given volume, and attempt to. We believe you with reference to refer? Burton's Microbiology for standing Health Sciences. -

Difference Between Chemoorganotrophic and Obligate Autotroph
Difference Between Chemoorganotrophic And Obligate Autotroph Cary underbidding misanthropically. Ulric often demise parlous when conjugated Ford mollycoddles indistinctly and domes her cyma. Lew often descants unfeignedly when isocheimic Tedie turn-offs designedly and Judaizing her accentors. No mechanism for anaerobic metabolism in conjunction with a common when algae, along the catabolism ofaromatic compounds provide evidence was a difference between trees inferred with the four genera have a consensus approach to The physiological characteristics of use different types of bacteria and their interactions. CO2 stimulates the chemoorganotrophic growth of both ammonia oxidizers and the. Chemoorganotrophic Definition of Chemoorganotrophic by. While Acidithiobacillus ferrooxidans growing on FeSO4 would depict an obligate aerobe. A fundamental metabolic distinction is between autotrophs and heterotrophs. Cthe intermediate steps of autotroph and only select multiple curvature and to safely place in each student need to a membrane that can be essentially the deep. The facultative autotroph also oxidizes a ridicule of organic compounds such. Bacterial Metabolism Medical Microbiology NCBI Bookshelf. Interestingly the amount cure the carboxylase in M capsulatus in chemostat culture. Facultative autotroph Can reproduce as autotroph if they must first better as. Learning Objectives Differentiate photoautotrophs from photoheterotrophs. Electron Transport Chain. Ecology of Cyanobacteria II Their Diversity in Space faculty Time. A Photographic Atlas for the Microbiology Laboratory. 5 Algae Biology LibreTexts. MB302 Oregon State University. Autotroph t-trf An organism that manufactures its own value from inorganic substances such high carbon dioxide and ammonia Most autotrophs such clean green plants certain algae and photosynthetic bacteria use ticket for energy. Ment was achieved by comparison by the labour count helpless the pervert of Thiobacillus A2 obtained on. -

Module 3 Microbial Metabolism
Module 3 Microbial Metabolism © 2013 Pearson Education, Inc. Chapter 5 Microbial Metabolism © 2013 Pearson Education, Inc. Lectures prepared by Helmut Kae Lectures prepared by Christine L. Case Copyright © 2013 Pearson Education, Inc. Catabolic and Anabolic Reactions § Metabolism: the sum of the chemical reactions in an organism § Two general types of metabolic reactions § Catabolism: provides energy and building blocks for anabolism § Anabolism: uses energy and building blocks to build large molecules § Recall from Chapter 2 § Energy can be stored when covalent bonds form § Energy can be released when covalent bonds broken © 2013 Pearson Education, Inc. Catabolic Reactions § Breakdown of complex substances to small molecules § Degradative reactions § Breaking of covalent bonds § What type of reaction used to break covalent bonds? § Purpose is to generate energy, make building blocks § Releases energy stored in bonds § Breakdown products used to build new molecules § Eg, breakdown of glucose for energy © 2013 Pearson Education, Inc. Anabolic Reactions § Building macromolecules from small molecules § Biosynthetic reactions § Forming of covalent bonds § What type of reaction used to form covalent bonds? § Requires energy to form bonds § Purpose is to generate materials for cell growth § Eg, making proteins from amino acids © 2013 Pearson Education, Inc. Figure 5.1 The role of ATP in coupling anabolic and catabolic reactions. Catabolism releases energy by oxidation of molecules Energy is Energy is released by stored in hydrolysis molecules of ATP of ATP Anabolism uses energy to synthesize macromolecules that make up the cell © 2013 Pearson Education, Inc. Catabolic and Anabolic Reactions § A metabolic pathway is a sequence of chemical reactions in a cell § Metabolic pathways are controlled by enzymes © 2013 Pearson Education, Inc. -

Phototrophic Methane Oxidation in a Member of the Chloroflexi Phylum
bioRxiv preprint doi: https://doi.org/10.1101/531582; this version posted January 26, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Phototrophic Methane Oxidation in a Member of the Chloroflexi Phylum Lewis M. Ward1,2, Patrick M. Shih3,4, James Hemp5, Takeshi Kakegawa6, Woodward W. Fischer7, Shawn E. McGlynn2,8,9 1. Department of Earth & Planetary Sciences, Harvard University, Cambridge, MA USA. 2. Earth-Life Science Institute, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo, Japan. 3. Department of Plant Biology, University of California, Davis, Davis, CA USA. 4. Department of Energy, Joint BioEnergy Institute, Emeryville, CA USA. 5. School of Medicine, University of Utah, Salt Lake City, UT USA. 6. Department of Geosciences, Tohoku University, Sendai City, Japan 7. Division of Geological & Planetary Sciences, California Institute of Technology, Pasadena, CA USA. 8. Biofunctional Catalyst Research Team, RIKEN Center for Sustainable Resource Science, Wako-shi Japan 9. Blue Marble Space Institute of Science, Seattle, WA, USA Abstract: Biological methane cycling plays an important role in Earth’s climate and the global carbon cycle, with biological methane oxidation (methanotrophy) modulating methane release from numerous environments including soils, sediments, and water columns. Methanotrophy is typically coupled to aerobic respiration or anaerobically via the reduction of sulfate, nitrate, or metal oxides, and while the possibility of coupling methane oxidation to phototrophy (photomethanotrophy) has been proposed, no organism has ever been described that is capable of this metabolism.