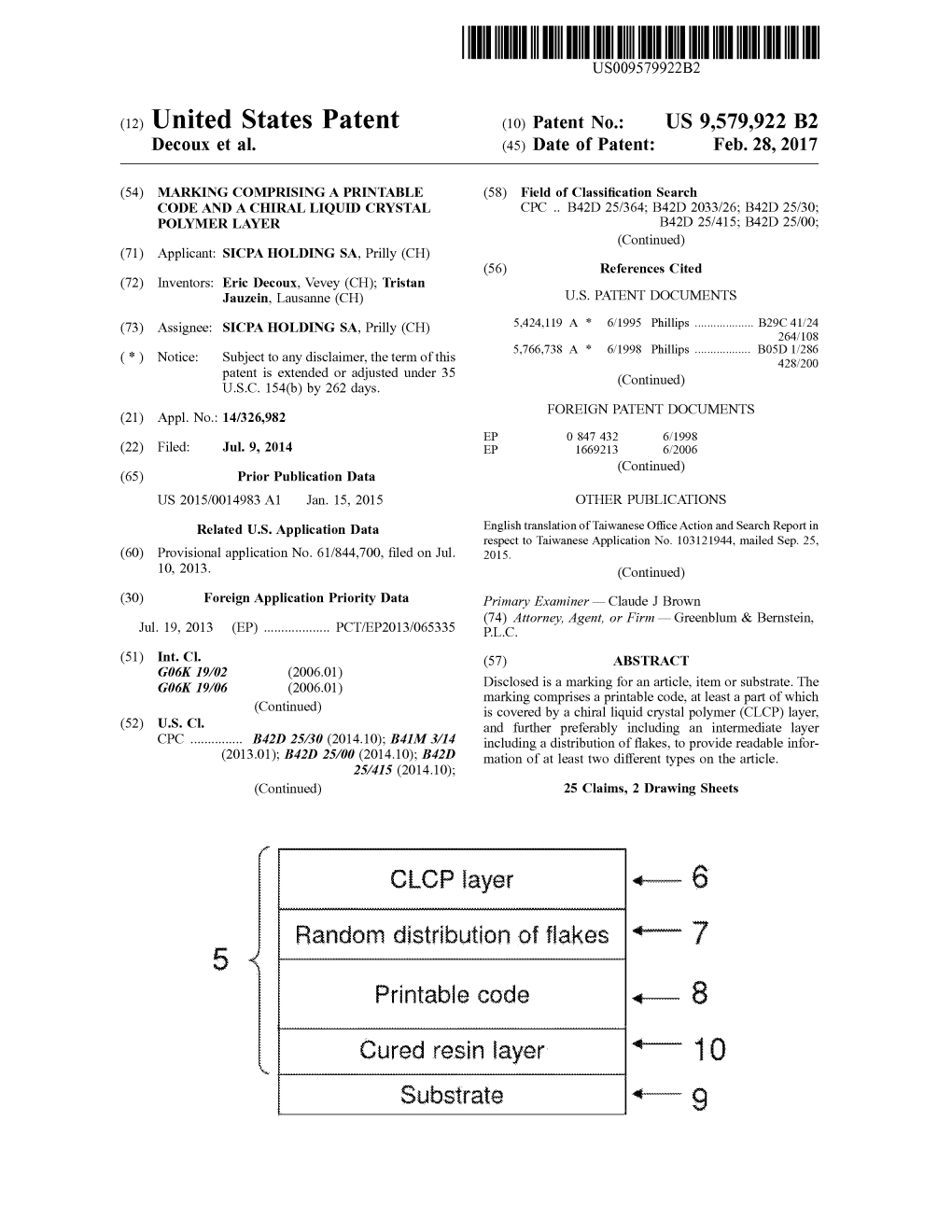
Cured Resin Layer - 10 Substrate 1-G US 9,579,922 B2 Page 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

List of New Mineral Names: with an Index of Authors
415 A (fifth) list of new mineral names: with an index of authors. 1 By L. J. S~v.scs~, M.A., F.G.S. Assistant in the ~Iineral Department of the,Brltish Museum. [Communicated June 7, 1910.] Aglaurito. R. Handmann, 1907. Zeita. Min. Geol. Stuttgart, col. i, p. 78. Orthoc]ase-felspar with a fine blue reflection forming a constituent of quartz-porphyry (Aglauritporphyr) from Teplitz, Bohemia. Named from ~,Xavpo~ ---- ~Xa&, bright. Alaito. K. A. ~Yenadkevi~, 1909. BuU. Acad. Sci. Saint-P6tersbourg, ser. 6, col. iii, p. 185 (A~am~s). Hydrate~l vanadic oxide, V205. H~O, forming blood=red, mossy growths with silky lustre. Founi] with turanite (q. v.) in thct neighbourhood of the Alai Mountains, Russian Central Asia. Alamosite. C. Palaehe and H. E. Merwin, 1909. Amer. Journ. Sci., ser. 4, col. xxvii, p. 899; Zeits. Kryst. Min., col. xlvi, p. 518. Lead recta-silicate, PbSiOs, occurring as snow-white, radially fibrous masses. Crystals are monoclinic, though apparently not isom0rphous with wol]astonite. From Alamos, Sonora, Mexico. Prepared artificially by S. Hilpert and P. Weiller, Ber. Deutsch. Chem. Ges., 1909, col. xlii, p. 2969. Aloisiite. L. Colomba, 1908. Rend. B. Accad. Lincei, Roma, set. 5, col. xvii, sere. 2, p. 233. A hydrated sub-silicate of calcium, ferrous iron, magnesium, sodium, and hydrogen, (R pp, R',), SiO,, occurring in an amorphous condition, intimately mixed with oalcinm carbonate, in a palagonite-tuff at Fort Portal, Uganda. Named in honour of H.R.H. Prince Luigi Amedeo of Savoy, Duke of Abruzzi. Aloisius or Aloysius is a Latin form of Luigi or I~ewis. -

A Review on Historical Earth Pigments Used in India's Wall Paintings
heritage Review A Review on Historical Earth Pigments Used in India’s Wall Paintings Anjali Sharma 1 and Manager Rajdeo Singh 2,* 1 Department of Conservation, National Museum Institute, Janpath, New Delhi 110011, India; [email protected] 2 National Research Laboratory for the Conservation of Cultural Property, Aliganj, Lucknow 226024, India * Correspondence: [email protected] Abstract: Iron-containing earth minerals of various hues were the earliest pigments of the prehistoric artists who dwelled in caves. Being a prominent part of human expression through art, nature- derived pigments have been used in continuum through ages until now. Studies reveal that the primitive artist stored or used his pigments as color cakes made out of skin or reeds. Although records to help understand the technical details of Indian painting in the early periodare scanty, there is a certain amount of material from which some idea may be gained regarding the methods used by the artists to obtain their results. Considering Indian wall paintings, the most widely used earth pigments include red, yellow, and green ochres, making it fairly easy for the modern era scientific conservators and researchers to study them. The present knowledge on material sources given in the literature is limited and deficient as of now, hence the present work attempts to elucidate the range of earth pigments encountered in Indian wall paintings and the scientific studies and characterization by analytical techniques that form the knowledge background on the topic. Studies leadingto well-founded knowledge on pigments can contribute towards the safeguarding of Indian cultural heritage as well as spread awareness among conservators, restorers, and scholars. -

Book Reviews
1127 The Canadian Mineralogist Vol. 43, pp. 1127-1128 (2005) BOOK REVIEWS Crystals: Growth, Morphology and Perfection by I. Chapter 5 (Surface microtopography of crystal faces). Sunagawa, Cambridge University Press, Cambridge, Chapter 6 (Perfections and homogeneity of single U.K., 2005, 295 + xii pages. CAN $127.95. Hardbound. crystals) gives a condensed exposition on the subject, ISBN 0–521–841–895. including “anatomy” phenomena in crystals, i.e., growth banding, growth sectors, and dislocations. Special The English version of this book has been prepared interest is paid in Chapter 7 (Regular intergrowth of based on the authorʼs translation, with a few modi- crystals) to twinning, parallel intergrowth, epitaxy, fications, of the Japanese version (2003; Kyoritsu exsolution, and some other cases of regular crystallo- Publishing Co.). The main purpose is to treat the subject graphic orientation between two or more single crystals of perfection and variability of crystal forms in the of the same or of different species. A short Chapter 8 mineral kingdom based on the laws of symmetry and (Forms and textures of polycrystalline aggregates) gives the conditions and mechanisms of crystal growth. The an overview of the rich variety of mineral aggregates, monograph includes 14 chapters incorporated in two including development of texture in multicomponent parts: Part I covers Fundamental Concepts (chapters 1– systems. 8, 163 pages), and Part II covers Application to Compli- cated and Complex Systems; Case Studies (chapters The case studies in Part II deal with single mineral 9–14, 112 pages). After each chapter, the author cites species such as diamond (Chapter 9), rock-crystal references to fundamental works. -

Fluid Inclusions in Fibrous and Octahedrally-Grown Diamonds
FLUID INCLUSIONS IN FIBROUS AND OCTAHEDRALLY-GROWN DIAMONDS by Evan Mathew Smith A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate and Postdoctoral Studies (Geological Sciences) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) March 2014 © Evan Mathew Smith, 2014 Abstract My thesis puts forth new models for diamond formation that explain the difference between octahedral and fibrous diamond growth, as well as the difference between octahedral diamond growth in the lithospheric and the sublithospheric mantle. Diamond growth in the mantle involves reactions between carbon-bearing fluid and the host rocks it infiltrates. This fluid is sometimes included in diamond. Fluids in dendritically-grown, fibrous diamonds from Wawa, Superior craton, were analysed in a novel way, using transmission X-ray diffraction. The technique allows bulk analysis of daughter minerals within fluid inclusions. The mineralogy, major and trace elements, Sr isotopes, volatiles, and nitrogen characteristics of the hydrous saline–high-Mg carbonatitic fluid in these Archean diamonds strongly resemble those of Phanerozoic fibrous diamonds. This implies that some mantle processes, including the formation of fibrous diamonds, can be extended unvaryingly back to 2.7 Ga. Fluid equilibrated with octahedrally-grown diamonds from the Siberian, Kaapvaal, and Congo cratons is trapped in healed fractures in the diamonds. They contain anhydrous CO2–N2 fluid inclusions with 40±4 mol% N2 and inclusions of former silicate melt that had an original N2 content of ~0.1 wt%, as shown by Raman, electron microprobe, and microthermometry analyses. The liberation of N2 from the convecting mantle is proposed to be controlled by increasing oxygen fugacity that destabilizes host phases. -

A Specific Gravity Index for Minerats
A SPECIFICGRAVITY INDEX FOR MINERATS c. A. MURSKyI ern R. M. THOMPSON, Un'fuersityof Bri.ti,sh Col,umb,in,Voncouver, Canad,a This work was undertaken in order to provide a practical, and as far as possible,a complete list of specific gravities of minerals. An accurate speciflc cravity determination can usually be made quickly and this information when combined with other physical properties commonly leads to rapid mineral identification. Early complete but now outdated specific gravity lists are those of Miers given in his mineralogy textbook (1902),and Spencer(M,i,n. Mag.,2!, pp. 382-865,I}ZZ). A more recent list by Hurlbut (Dana's Manuatr of M,i,neral,ogy,LgE2) is incomplete and others are limited to rock forming minerals,Trdger (Tabel,l,enntr-optischen Best'i,mmungd,er geste,i,nsb.ildend,en M,ineral,e, 1952) and Morey (Encycto- ped,iaof Cherni,cal,Technol,ogy, Vol. 12, 19b4). In his mineral identification tables, smith (rd,entifi,cati,onand. qual,itatioe cherai,cal,anal,ys'i,s of mineral,s,second edition, New york, 19bB) groups minerals on the basis of specificgravity but in each of the twelve groups the minerals are listed in order of decreasinghardness. The present work should not be regarded as an index of all known minerals as the specificgravities of many minerals are unknown or known only approximately and are omitted from the current list. The list, in order of increasing specific gravity, includes all minerals without regard to other physical properties or to chemical composition. The designation I or II after the name indicates that the mineral falls in the classesof minerals describedin Dana Systemof M'ineralogyEdition 7, volume I (Native elements, sulphides, oxides, etc.) or II (Halides, carbonates, etc.) (L944 and 1951). -

The Crystal Structure of the Mineral Scholzite and a Study of the Crystal
\ I 7'1,71 ¡1 :), THE CRYSTAL STRUCTURE OF THE MINERAL SCHOLZITE AND A STUDY OF THE CRYSTAL CHEMISTRY OF SOME RELATED PHOSPHATE AND ARSENATE MINERALS A thesis submitted for the Degree of Doctor of PhilosoPhY at the University of Adelaide in April, 1975 by R0DERICK JEFFREY HILL, B.Sc. (Hons.) Department of Geology and Mineralogy Au/¿tr¡'t ,,! /'/,".'','-'"' ' TABLE OF CONTENTS Page SUMMARY (i) STATEMENT OF ORIGINATITY (ii) ACKNOWLEDGEMENTS (iii) GENERAL INTRODUCTION I CHAPTER 1 TIIE GEOI.OGY AbID MINERALOGY OF REAPHOOK HILL, SOUTTI AUSTR.ALIA 2 1.1 ABSTR,ACT 2 r.2 INTRODUCTTON 2 1.3 GEOIÐGICAL SETTING 3 I.4 EXPERTMENTAI TECHNIQT ES 5 1.5 THE MAJOR PHOSPHATE MTNERALS 6 1.5.1 Tarbuttite - Znr(po4) (OH) 6 1.5.2 Parahopeite ZnrZn(pOn) - Z.4HZo 9 I.5.3 Scholzite CaZnU(pO4) - 2.2H2O 9 1.5.4 Zincian Collinsite Car(Mg,Zn) (pO4) - 2.2H2O 15 1.6 ASPECTS OF EHE CRYSTA¡ CHEMISTRY OF THE MAJOR PHOSPHATE MINERALS 19 L.7 PARAGENESIS 23 1.7.1 Major Minerals 23 L.7.2 Other lvlinerals 26 1.7.3 Conclusions 27 CHAPTER 2 TIIE CRYSTAI STRUCTURE OF SCHOLZITE 30 2.L ABSTRACT 30 2.2 INTRODUCTION 32 2.3 DATA COLLECTION AND ÐATA REDUCTION 32 2.4 DISCUSSION OF TITE INTENSITY DISTRIBUTION 35 2.4.L Subcell Structure and pseudosynunetry 35 2.4.2 Dete::mination of the True Syrnmetry 4L 2.4.3 Structural Disorder 4l 2.5 STRUqrURE SOLUTION AND REFINEMENT OF THE AVER.AGE ST'BCELL 52 2.6 DESCRIPTION AND DISCUSSION OF THE AVERAGE ST]BCELL STRUCTURE 60 2.6.I Topology 60 2.6.2 Disorder 65 2.6.3'iThermal" parameters 67 2.7 STRUCTURE SOLUTION AND REFTNEMENT OF TITE },IAIN CELL -

The Vibrational Spectroscopy of Minerals
THE VIBRATIONAL SPECTROSCOPY OF MINERALS WAYDE NEIL MARTENS B. APPL. SCI. (APPL. CHEM.) M.SC. (APPL. SCI.) Inorganic Materials Research Program, School of Physical and Chemical Science, Queensland University of Technology A THESIS SUBMITED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY OF THE QUEENSLAND UNIVERSITY OF TECHNOLOGY 2004 2 Toss another rock on the Raman… 3 KEYWORDS Annabergite Aragonite Arupite Baricite Cerussite Erythrite Hörnesite Infrared Spectroscopy Köttigite Minerals Parasymplesite Raman Spectroscopy Solid Solutions Strontianite Vibrational Spectroscopy Vivianite Witherite 4 ABSTRACT This thesis focuses on the vibrational spectroscopy of the aragonite and vivianite arsenate minerals (erythrite, annabergite and hörnesite), specifically the assignment of the spectra. The infrared and Raman spectra of cerussite have been assigned according to the vibrational symmetry species. The assignment of satellite bands to 18O isotopes has been discussed with respect to the use of these bands to the quantification of the isotopes. Overtone and combination bands have been assigned according to symmetry species and their corresponding fundamental vibrations. The vibrational spectra of cerussite have been compared with other aragonite group minerals and the differences explained on the basis of differing chemistry and crystal structures of these minerals. The single crystal spectra of natural erythrite has been reported and compared with the synthetic equivalent. The symmetry species of the vibrations have been assigned according to single crystal and factor group considerations. Deuteration experiments have allowed the assignment of water vibrational frequencies to discrete water molecules in the crystal structure. Differences in the spectra of other vivianite arsenates, namely annabergite and hörnesite, have been explained by consideration of their differing chemistry and crystal structures. -

Antoine 2 HAL.Pdf
Trace element carriers in combined sewer during dry and wet weather: an electron microscope investigation A.G El Samrani, B.S Lartiges, J. Ghanbaja, J. Yvon, A Kohler To cite this version: A.G El Samrani, B.S Lartiges, J. Ghanbaja, J. Yvon, A Kohler. Trace element carriers in combined sewer during dry and wet weather: an electron microscope investigation. Water Research, IWA Publishing, 2004, 38 (8), pp.2063-2076. 10.1016/j.watres.2004.01.029. hal-02283422 HAL Id: hal-02283422 https://hal.archives-ouvertes.fr/hal-02283422 Submitted on 10 Sep 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. TRACE ELEMENT CARRIERS IN COMBINED SEWER DURING DRY AND WET WEATHER: AN ELECTRON MICROSCOPE INVESTIGATION A.G. El Samrani*, B. S. Lartiges*(a), J. Ghanbaja‡, J. Yvon*, A. Kohler‡ *Laboratoire Environnement et Minéralurgie (LEM-ENSG) Pôle de l’Eau 15, Avenue du Charmois –BP 40 – 54 501 Vandœuvre, FRANCE ‡ Université Henri Poincaré - Service Commun de Microscopie Electronique BP 239. 54 500 Vandœuvre Cedex, FRANCE. E-mail: [email protected] (a) To whom correspondence should be addressed ABSTRACT The nature of trace element carriers contained in sewage and combined sewer overflow (CSO) was investigated by TEM-EDX-Electron diffraction and SEM-EDX. -

Download the Scanned
CLASSIFICATION OF MINERALS OF THE TYPE fu(XO a)2' nH2O ( Concluded) C. W. Wornn, Harvard. Uniaersity, Combri.d.ge,Mass. \LonnnuedJrjn pdge/)J) CoNrnNrs or Penr 2 Page The Ar(XOr)z 4HzO Family. 787 Parahcpeite .. 788 Anapaite 788 Messelite 790 Phosphophyllite 792 The Relations of Phosphophyllite to Hcpeite 79s Hopeite. 795 Trichalcite 799 The As(XOr)z SHrO Family 800 Choice of Unit Cell 800 Optical Properties 801 Chemistry , 801 Symplesite. 801 Vivianite. 803 Annabergite. 804 Erythrite. 804 K6ttigite. 804 Bobierrite 806 Iloernesite 806 Conclusions 807 Ack nowledgments. 808 Bibliography 808 fnn fu (XO+)z. 4HzO Fenrr,y This family is composed of the triclinic group-parahopeite and ana- paite; the monoclinic member phosphophyllite; and the orthorhombic member-hopeite. The relations between the unit cells of phosphophyl- lite and hopeite are simple and are given in the description of the former. Parahopeite and anapaite have very similar unit cells, their differences being due solely to the variation in cation content. The relation between the cells of the triclinic members and the monoclinic and orthorhombic membersis not clear. The addition of one or more molecules of water to each crystallizing molecule must be accompanied by a completely new bonding arrange- ment, for there is no recognizablerelation between the unit cells of the members of the various families and there is no single factor of the unit cells which varies with the water variation. Thus it is impossible to relate the unit cells of the various families, although there is a definite relation 787 788 C. W. WOLFE between the cell volumes and the number of water molecules. -

The Microscopic Determination of the Nonopaque Minerals
DEPARTMENT OF THE INTERIOR ALBERT B. FALL, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 679 THE MICROSCOPIC DETERMINATION OF THE NONOPAQUE MINERALS BY ESPER S. LARSEN WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CONTENTS. CHAPTER I. Introduction.................................................. 5 The immersion method of identifying minerals........................... 5 New data............................................................. 5 Need of further data.................................................... 6 Advantages of the immersion method.................................... 6 Other suggested uses for the method.................................... 7 Work and acknowledgments............................................. 7 CHAPTER II. Methods of determining the optical constants of minerals ....... 9 The chief optical constants and their interrelations....................... 9 Measurement of indices of refraction.................................... 12 The embedding method............................................ 12 The method of oblique illumination............................. 13 The method of central illumination.............................. 14 Immersion media.................................................. 14 General features............................................... 14 Piperine and iodides............................................ 16 Sulphur-selenium melts....................................... 38 Selenium and arsenic selenide melts........................... 20 Methods of standardizing -

AFMS Mineral List 2003
American Federation Of Mineralogical Societies AFMS Mineral Classification List New Edition Updated for 2003 AFMS Publications Committee B. Jay Bowman, Chair 1 Internet version of Mineral Classification List. This document may only be downloaded at: http://www.amfed.org/rules/ Introduction to the Mineral Classification List The AFMS Rules Committee voted to eliminate the listing in the Rulebook of references for mineral names except for the AFMS Mineral Classification List. Exhibitors are encouraged to use the AFMS List when exhibiting in the B Division (Minerals). If the mineral they are exhibiting is not on the AFMS List they should note on the Mineral list they present to the judging chairman which reference they did use for the information on their label. The Regional Rules Chairs have been asked to submit names to be added to the list which will be updated with addendum’s each year. In a few years the list should represent most of the minerals generally exhibited out of the 4200+ now recognized by the IMA. This list follows the Glossary of Mineral species which is the IMA approved names for minerals. When the Official name of the mineral includes diacritical mark, they are underlined to indicate they are the IMA approved name. Where usage of old names has been in use for years they have been included, but with the approved spelling underlined following it. The older spelling will be accepted for the present so exhibitors will not have to correct there present label. This list may not contain all mineral species being exhibited. Exhibitors are encouraged to submit names to be added to the list to the Rules committee.