The Pharmacokinetics and Pharmacodynamics Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

268 Part 522—Implantation Or Injectable Dosage Form
§ 520.2645 21 CFR Ch. I (4–1–18 Edition) (ii) Indications for use. For the control 522.82 Aminopropazine. of American foulbrood (Paenibacillus 522.84 Beta-aminopropionitrile. larvae). 522.88 Amoxicillin. 522.90 Ampicillin injectable dosage forms. (iii) Limitations. The drug should be 522.90a Ampicillin trihydrate suspension. fed early in the spring or fall and con- 522.90b Ampicillin trihydrate powder for in- sumed by the bees before the main jection. honey flow begins, to avoid contamina- 522.90c Ampicillin sodium. tion of production honey. Complete 522.144 Arsenamide. treatments at least 4 weeks before 522.147 Atipamezole. main honey flow. 522.150 Azaperone. 522.161 Betamethasone. [40 FR 13838, Mar. 27, 1975, as amended at 50 522.163 Betamethasone dipropionate and FR 49841, Dec. 5, 1985; 59 FR 14365, Mar. 28, betamethasone sodium phosphate aque- 1994; 62 FR 39443, July 23, 1997; 68 FR 24879, ous suspension. May 9, 2003; 70 FR 69439, Nov. 16, 2005; 73 FR 522.167 Betamethasone sodium phosphate 76946, Dec. 18, 2008; 75 FR 76259, Dec. 8, 2010; and betamethasone acetate. 76 FR 59024, Sept. 23, 2011; 77 FR 29217, May 522.204 Boldenone. 17, 2012; 79 FR 37620, July 2, 2014; 79 FR 53136, 522.224 Bupivacaine. Sept. 8, 2014; 79 FR 64116, Oct. 28, 2014; 80 FR 522.230 Buprenorphine. 34278, June 16, 2015; 81 FR 48702, July 26, 2016] 522.234 Butamisole. 522.246 Butorphanol. § 520.2645 Tylvalosin. 522.275 N-Butylscopolammonium. 522.300 Carfentanil. (a) Specifications. Granules containing 522.304 Carprofen. 62.5 percent tylvalosin (w/w) as 522.311 Cefovecin. -

Sample Chapter
LCMS_Chap09 (JB-D) 8/5/06 3:14 pm Page 193 9 LC-MS in doping control Detlef Thieme Introduction adjacent fields with similar analytical prospects, like veterinary residue control (predominantly dealing with identification of growth promoters Definition of doping in various matrices), forensic sciences (the major- ity of doping-relevant substances are scheduled Doping analysis comprises a diversity of sub- as controlled substances in most countries), stance classes with different pharmaceutical and environmental analysis (e.g. steroids in waste chemical properties. Therefore, the discussion of water) or clinical chemistry (e.g. due to the the suitability of liquid chromatography-mass increasing relevance of steroid hormone replace- spectrometry (LC-MS) in doping analysis needs ment therapy). to distinguish various categories. This chapter describes the key fields of appli- According to its formal definition, a doping cation of LC-MS in routine doping control (i.e. violation in sports can be caused by various screening analysis, confirmation and quantifica- events, e.g.: tion of positive results) extra to particular • the detection of a prohibited substance or research activities. The latter are focused on the metabolites or markers of that substance (as intended technical improvements (e.g. extension defined by the recent document [1] of the of detection time windows, reduction of turn- World Anti-Doping Agency [WADA]) in the around times and costs) of conventional analyti- athlete’s specimen cal procedures and, in particular, on the detection • the use of prohibited substances or methods of prohibited substances that cannot be ade- • possession or trafficking prohibited substances quately identified so far (e.g. -
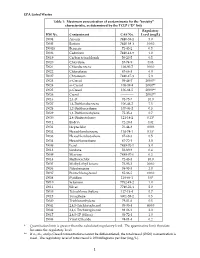
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

DIURETICS Diuretics Are Drugs That Promote the Output of Urine Excreted by the Kidneys
DIURETICS Diuretics are drugs that promote the output of urine excreted by the Kidneys. The primary action of most diuretics is the direct inhibition of Na+ transport at one or more of the four major anatomical sites along the nephron, where Na+ reabsorption takes place. The increased excretion of water and electrolytes by the kidneys is dependent on three different processes viz., glomerular filtration, tubular reabsorption (active and passive) and tubular secretion. Diuretics are very effective in the treatment of Cardiac oedema, specifically the one related with congestive heart failure. They are employed extensively in various types of disorders, for example, nephritic syndrome, diabetes insipidus, nutritional oedema, cirrhosis of the liver, hypertension, oedema of pregnancy and also to lower intraocular and cerebrospinal fluid pressure. Therapeutic Uses of Diuretics i) Congestive Heart Failure: The choice of the diuretic would depend on the severity of the disorder. In an emergency like acute pulmonary oedema, intravenous Furosemide or Sodium ethacrynate may be given. In less severe cases. Hydrochlorothiazide or Chlorthalidone may be used. Potassium-sparing diuretics like Spironolactone or Triamterene may be added to thiazide therapy. ii) Essential hypertension: The thiazides usually sever as primary antihypertensive agents. They may be used as sole agents in patients with mild hypertension or combined with other antihypertensives in more severe cases. iii) Hepatic cirrhosis: Potassium-sparing diuretics like Spironolactone may be employed. If Spironolactone alone fails, then a thiazide diuretic can be added cautiously. Furosemide or Ethacrymnic acid may have to be used if the oedema is regractory, together with spironolactone to lessen potassium loss. Serum potassium levels should be monitored periodically. -

Uniform Classification Guidelines for Foreign Substances and Recommended Penalties Model Rule
DRUG TESTING STANDARDS AND PRACTICES PROGRAM. Uniform Classification Guidelines for Foreign Substances And Recommended Penalties Model Rule. January, 2018 (V.13.4) Ó ASSOCIATION OF RACING COMMISSIONERS INTERNATIONAL – 2018. Association of Racing Commissioners International 1510 Newtown Pike, Lexington, Kentucky, United States www.arci.com Page 1 of 61 Preamble to the Uniform Classification Guidelines of Foreign Substances The Preamble to the Uniform Classification Guidelines was approved by the RCI Drug Testing and Quality Assurance Program Committee (now the Drug Testing Standards and Practices Program Committee) on August 26, 1991. Minor revisions to the Preamble were made by the Drug Classification subcommittee (now the Veterinary Pharmacologists Subcommittee) on September 3, 1991. "The Uniform Classification Guidelines printed on the following pages are intended to assist stewards, hearing officers and racing commissioners in evaluating the seriousness of alleged violations of medication and prohibited substance rules in racing jurisdictions. Practicing equine veterinarians, state veterinarians, and equine pharmacologists are available and should be consulted to explain the pharmacological effects of the drugs listed in each class prior to any decisions with respect to penalities to be imposed. The ranking of drugs is based on their pharmacology, their ability to influence the outcome of a race, whether or not they have legitimate therapeutic uses in the racing horse, or other evidence that they may be used improperly. These classes of drugs are intended only as guidelines and should be employed only to assist persons adjudicating facts and opinions in understanding the seriousness of the alleged offenses. The facts of each case are always different and there may be mitigating circumstances which should always be considered. -

Mechanisms of Sevoflurane-Induced Myocardial Preconditioning In
Anesthesiology 2003; 99:27–33 © 2003 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Mechanisms of Sevoflurane-induced Myocardial Preconditioning in Isolated Human Right Atria In Vitro Alexandra Yvon, B.Sc.,* Jean-Luc Hanouz, M.D, Ph.D.,† Benoît Haelewyn, B.Sc.,* Xavier Terrien, B.Sc.,* Massimo Massetti, M.D.,‡ Gérard Babatasi, M.D., Ph.D.,‡ André Khayat, M.D.,§ Pierre Ducouret, Ph.D., Henri Bricard, M.D.,# Jean-Louis Gérard, M.D., Ph.D.** Background: The authors examined the role of adenosine postischemic contractile function,5–7 and metabolic triphosphate–sensitive potassium channels and adenosine A 7 1 function. Although the mechanisms involved in anes- receptors in sevoflurane-induced preconditioning on isolated thetic preconditioning remain incompletely understood, human myocardium. Methods: The authors recorded isometric contraction of hu- protein kinase C, adenosine triphosphate–sensitive po- man right atrial trabeculae suspended in oxygenated Tyrode’s tassium (KATP) channels, and adenosine A1 receptors Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/99/1/27/408853/0000542-200307000-00008.pdf by guest on 02 October 2021 solution (34°C; stimulation frequency, 1 Hz). In all groups, a seem to play a key role.1,2,5,6 However, recent studies 30-min hypoxic period was followed by 60 min of reoxygenation. suggest that volatile anesthetic–induced myocardial pre- Seven minutes before hypoxia reoxygenation, muscles were ex- posed to 4 min of hypoxia and 7 min of reoxygenation or 15 min conditioning may be dependent on species and experi- 5,8 of sevoflurane at concentrations of 1, 2, and 3%. In separate mental models. -

Differential Regulation of Endothelium Behavior by Progesterone and Medroxyprogesterone Acetate
P H CUTINI and others Progestins and vascular function 220:3 179–193 Research Differential regulation of endothelium behavior by progesterone and medroxyprogesterone acetate Pablo H Cutini1,2, Adria´n E Campelo1,2 and Virginia L Massheimer1,2 Correspondence should be addressed 1Ca´ tedra de Bioquı´mica Clı´nica II, Departamento de Biologı´a, Bioquı´mica y Farmacia, Universidad Nacional to V L Massheimer del Sur (UNS), San Juan 670, B8000ICN, Bahı´a Blanca, Argentina Email 2Consejo Nacional de Investigaciones Cientı´ficas y Te´ cnicas (CONICET), Argentina, Buenos Aires, Argentina [email protected] Abstract Medroxyprogesterone acetate (MPA) is a synthetic progestin commonly used in hormone Key Words replacement therapy (HRT). The aim of this research was to study and compare the effect of " cell migration progesterone (Pg) and MPA on the regulation of cellular events associated with vascular " medroxyprogesterone homeostasis and disease. Platelet adhesion to endothelial cells (ECs), nitric oxide (NO) acetate production, and cell migration were studied using murine ECs in vitro exposed to the " nitric oxide progestins. After 7 min of treatment, MPA significantly inhibited NO synthesis with respect " progesterone to control values; meanwhile, Pg markedly increased vasoactive production. In senile ECs, " vascular tissue the stimulatory action of Pg decreases; meanwhile, MPA maintained its ability to inhibit Journal of Endocrinology NO synthesis. The presence of RU486 antagonized the action of each steroid. When ECs were preincubated with PD98059 (MAPK inhibitor) or chelerythrine (protein kinase C (PKC) inhibitor) before Pg or MPA treatment, the former totally suppressed the steroid action, but the PKC antagonist did not affect NO production. -

FAST FACTS and CONCEPTS #20 OPIOID DOSE ESCALATION in END-OF-LIFE CARE David E Weissman MD, Andrew Kamell MD Background a Comm
FAST FACTS AND CONCEPTS #20 OPIOID DOSE ESCALATION IN END-OF-LIFE CARE David E Weissman MD, Andrew Kamell MD Background A common question from trainees is “How fast, and by how much, can opioids be safely dose escalated in terminally ill patients?” Consider the analogy of furosemide (Lasix) when discussing this topic. An increase in Lasix from 10 mg to 11 mg is unheard of, yet that is precisely what often happens with opioids, especially parenteral infusions. For example, increasing a morphine infusion from 1 to 2 mg/hr is a 100% does increase; while going from 5 to 6 mg/hr is only a 20% increase. Yet, many orders are written, “increase drip by 1 mg/hr, titrate to comfort.” Some hospitals and nursing units even have this as a standing order or nursing policy. Such titration orders are unlikely to lead to be effective. Key clinical considerations for appropriate opioid escalation: 1. Is an opioid dose escalation appropriate? To answer that question, clinicians need to consider: a. Is the patient tolerating opioids well with little adverse effects? b. Is the pain or dyspnea expected to continue at a similar or progressive pattern (particularly relevant for scheduled medications or infusions) or might it be temporary? c. Is the source of discomfort opioid responsive? d. Is there evidence of hyperalgesia or neurotoxicity from opioids? See Fast Facts #57,58, and 142. e. Is the pain related to a chronic, non-malignant source in which the patient has an extended prognosis? If so, then aggressive opioid titrations may not be the best long-term solution. -

Progesterone Using ALZET Osmotic Pumps
ALZET® Bibliography References on the Administration of Progesterone Using ALZET Osmotic Pumps Q8558: V. Joseph, et al. Progesterone decreases apnoea and reduces oxidative stress induced by chronic intermittent hypoxia in ovariectomized female rats. Exp Physiol 2020;105(6):1025-1034 Agents: Progesterone Vehicle: Cyclodextrin, 2-ß-Hydroxypropl-; Route: SC; Species: Rat; Pump: 2ML4; Duration: 28 days; ALZET Comments: Dose (4 mg/kg/day); Controls received mp w/ vehicle; animal info (Sprague-Dawley female rats (220-250g/57-70 days old)); post op. care (buprenorphine); Blood pressure measured via tail cuff method;93.3 mmHg - 105.2 mmHg;Progesterone aka prog; dependence; Q6232: S. F. Rosen, et al. T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 2017;37(41):9819-9827 ALZET Comments: Estradiol, 17b-; Progesterone sulfate; SC; Mice; 2002; 14 days; Dose (17b-estradiol : 0.1 mg/kg/d, progesterone sulfate: 0.25 mg/kg/d, 0.1 mg/kg/d estradiol + 0.25 mg/kg/d progesterone); Controls received mp w/ vehicle; animal info (7-12 week old female C57BL/6J mice); replacement therapy (estradiol, ovariectomy); Therapeutic indication. Q6066: D. J. Morris, et al. Glucocorticoids and gut bacteria: "The GALF Hypothesis" in the metagenomic era. Steroids 2017;125(1-13 ALZET Comments: Chenodeoxycholic acid, progesterone, 11b-hydroxy-, corticosterone, deoxy-, corticosterone, 3α,5α-TH-, progesterone, 3α,5α-TH-11β-hydroxy-; SC; Rat; steroidal derivatives of corticosterone; Review presents the role of gut microbial metabolism of endogenous adrenocorticosteroids as a contributing factor in the etiology of essential hypertension. Q6204: S. McIlvride, et al. A progesterone-brown fat axis is involved in regulating fetal growth. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

2021 Formulary List of Covered Prescription Drugs
2021 Formulary List of covered prescription drugs This drug list applies to all Individual HMO products and the following Small Group HMO products: Sharp Platinum 90 Performance HMO, Sharp Platinum 90 Performance HMO AI-AN, Sharp Platinum 90 Premier HMO, Sharp Platinum 90 Premier HMO AI-AN, Sharp Gold 80 Performance HMO, Sharp Gold 80 Performance HMO AI-AN, Sharp Gold 80 Premier HMO, Sharp Gold 80 Premier HMO AI-AN, Sharp Silver 70 Performance HMO, Sharp Silver 70 Performance HMO AI-AN, Sharp Silver 70 Premier HMO, Sharp Silver 70 Premier HMO AI-AN, Sharp Silver 73 Performance HMO, Sharp Silver 73 Premier HMO, Sharp Silver 87 Performance HMO, Sharp Silver 87 Premier HMO, Sharp Silver 94 Performance HMO, Sharp Silver 94 Premier HMO, Sharp Bronze 60 Performance HMO, Sharp Bronze 60 Performance HMO AI-AN, Sharp Bronze 60 Premier HDHP HMO, Sharp Bronze 60 Premier HDHP HMO AI-AN, Sharp Minimum Coverage Performance HMO, Sharp $0 Cost Share Performance HMO AI-AN, Sharp $0 Cost Share Premier HMO AI-AN, Sharp Silver 70 Off Exchange Performance HMO, Sharp Silver 70 Off Exchange Premier HMO, Sharp Performance Platinum 90 HMO 0/15 + Child Dental, Sharp Premier Platinum 90 HMO 0/20 + Child Dental, Sharp Performance Gold 80 HMO 350 /25 + Child Dental, Sharp Premier Gold 80 HMO 250/35 + Child Dental, Sharp Performance Silver 70 HMO 2250/50 + Child Dental, Sharp Premier Silver 70 HMO 2250/55 + Child Dental, Sharp Premier Silver 70 HDHP HMO 2500/20% + Child Dental, Sharp Performance Bronze 60 HMO 6300/65 + Child Dental, Sharp Premier Bronze 60 HDHP HMO