Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Biology of Nitric Oxide, Part 7
Cell Death and Differentiation (2001) 8, 106 ± 108 ã 2001 Nature Publishing Group All rights reserved 1350-9047/01 $15.00 www.nature.com/cdd Book Review The Biology of Nitric Oxide, Part 7 B BruÈne University of Erlangen-NuÈrnberg, Medical Department IV, Experimental Division, Loschgestrasse 8, 91054 Erlangen, Germany The Biology of Nitric Oxide Part 7. Edited by S Moncada, LE Gustafsson, NP Wiklund, EA Higgs, Portland Press, London, UK: 2000, Pp. 234, £110, ISBN: 1 85578 142 5 This book is in the tradition of previously related titles (The covered by this book are quite extensive and an up-to-date Biology of Nitric Oxide, Parts 1 ± 6) that cover cutting edge summary of the current status of the field is provided. This scientific contribution related to the chemistry, biology, and is excellent for insiders, looking for detailed information, medicine of nitric oxide. Ten years after the initiating new cross-links, or just being interested in the current conference on a series of NO conventions, which was held research focus of individual groups. In this respect, most of in London in September 1989, this book now summarizes the chapters are well organized, providing background about 35 oral communications and approximately 300 poster information, technical comments, results, and a brief presentations of the 6th International Meeting on the Biology discussion. In most papers, figures or tables illustrate of Nitric Oxide, which was organized in September 1999 in major findings and references provide information for more Stockholm, Sweden. With the editors, especially Professor advanced reading on a particular topic. At the same time, Dr. -

Nitric Oxide Produced by Ultraviolet-Irradiated Keratinocytes Stimulates Melanogenesis
Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. C Roméro-Graillet, … , J P Ortonne, R Ballotti J Clin Invest. 1997;99(4):635-642. https://doi.org/10.1172/JCI119206. Research Article Ultraviolet (UV) radiation is the main physiological stimulus for human skin pigmentation. Within the epidermal-melanin unit, melanocytes synthesize and transfer melanin to the surrounding keratinocytes. Keratinocytes produce paracrine factors that affect melanocyte proliferation, dendricity, and melanin synthesis. In this report, we show that normal human keratinocytes secrete nitric oxide (NO) in response to UVA and UVB radiation, and we demonstrate that the constitutive isoform of keratinocyte NO synthase is involved in this process. Next, we investigate the melanogenic effect of NO produced by keratinocytes in response to UV radiation using melanocyte and keratinocyte cocultures. Conditioned media from UV-exposed keratinocytes stimulate tyrosinase activity of melanocytes. This effect is reversed by NO scavengers, suggesting an important role for NO in UV-induced melanogenesis. Moreover, melanocytes respond to NO-donors by decreased growth, enhanced dendricity, and melanogenesis. The rise in melanogenesis induced by NO-generating compounds is associated with an increased amount of both tyrosinase and tyrosinase-related protein 1. These observations suggest that NO plays an important role in the paracrine mediation of UV-induced melanogenesis. Find the latest version: https://jci.me/119206/pdf Nitric Oxide Produced by Ultraviolet-irradiated Keratinocytes Stimulates Melanogenesis Christine Roméro-Graillet, Edith Aberdam, Monique Clément, Jean-Paul Ortonne, and Robert Ballotti Institut National de la Santé et de la Recherche Médicale U385, Faculté de Médecine, 06107 Nice cedex 02, France Abstract lin-1 (ET-1),1 and GM-CSF by keratinocytes is upregulated after UV light exposure, and these peptides stimulate melanocyte Ultraviolet (UV) radiation is the main physiological stimu- growth (16–19). -
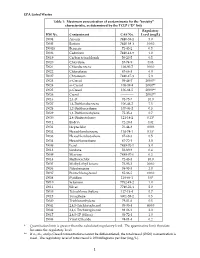
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Potential Roles of Nitrate and Nitrite in Nitric Oxide Metabolism in the Eye Ji Won Park1, Barbora Piknova1, Audrey Jenkins2, David Hellinga2, Leonard M
www.nature.com/scientificreports OPEN Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye Ji Won Park1, Barbora Piknova1, Audrey Jenkins2, David Hellinga2, Leonard M. Parver3 & Alan N. Schechter1* Nitric oxide (NO) signaling has been studied in the eye, including in the pathophysiology of some eye diseases. While NO production by nitric oxide synthase (NOS) enzymes in the eye has been − characterized, the more recently described pathways of NO generation by nitrate ( NO3 ) and nitrite − (NO2 ) ions reduction has received much less attention. To elucidate the potential roles of these pathways, we analyzed nitrate and nitrite levels in components of the eye and lacrimal glands, primarily in porcine samples. Nitrate and nitrite levels were higher in cornea than in other eye parts, while lens contained the least amounts. Lacrimal glands exhibited much higher levels of both ions compared to other organs, such as liver and skeletal muscle, and even to salivary glands which are known to concentrate these ions. Western blotting showed expression of sialin, a known nitrate transporter, in the lacrimal glands and other eye components, and also xanthine oxidoreductase, a nitrate and nitrite reductase, in cornea and sclera. Cornea and sclera homogenates possessed a measurable amount of nitrate reduction activity. These results suggest that nitrate ions are concentrated in the lacrimal glands by sialin and can be secreted into eye components via tears and then reduced to nitrite and NO, thereby being an important source of NO in the eye. Te NO generation pathway from L-arginine by endogenous NOS enzymes under normoxic conditions has been central in identifying the physiological roles of NO in numerous biological processes1. -

Antiproliferative Effects of Carbon Monoxide on Pancreatic Cancer
Digestive and Liver Disease 46 (2014) 369–375 Contents lists available at ScienceDirect Digestive and Liver Disease jou rnal homepage: www.elsevier.com/locate/dld Oncology Antiproliferative effects of carbon monoxide on pancreatic cancer a,b,∗ c,1 a a Libor Vítek , Helena Gbelcová , Lucie Muchová , Katerinaˇ Vánovᡠ, a,2 a a a Jaroslav Zelenka , Renata Koníckovᡠ, Jakub Sukˇ , Marie Zadinova , c d,e d d,e c,∗∗ Zdenekˇ Knejzlík , Shakil Ahmad , Takeshi Fujisawa , Asif Ahmed , Tomásˇ Ruml a Institute of Medical Biochemistry and Laboratory Diagnostics, 1st Faculty of Medicine, Charles University in Prague, Prague 2, Czech Republic b 4th Department of Internal Medicine, 1st Faculty of Medicine, Charles University in Prague, Prague 2, Czech Republic c Department of Biochemistry and Microbiology, Institute of Chemical Technology, Prague 6, Czech Republic d Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK e School of Life & Health Sciences, Aston University, Birmingham, UK a r t i c l e i n f o a b s t r a c t Article history: Background: Carbon monoxide, the gaseous product of heme oxygenase, is a signalling molecule with Received 14 June 2013 a broad spectrum of biological activities. The aim of this study was to investigate the effects of carbon Accepted 4 December 2013 monoxide on proliferation of human pancreatic cancer. Available online 14 January 2014 Methods: In vitro studies were performed on human pancreatic cancer cells (CAPAN-2, BxPc3, and PaTu- 8902) treated with a carbon monoxide-releasing molecule or its inactive counterpart, or exposed to Keywords: carbon monoxide gas (500 ppm/24 h). -

Nitric Oxide Signaling in Plants
plants Editorial Nitric Oxide Signaling in Plants John T. Hancock Department of Applied Sciences, University of the West of England, Bristol BS16 1QY, UK; [email protected]; Tel.: +44-(0)117-328-2475 Received: 3 November 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: Nitric oxide (NO) is an integral part of cell signaling mechanisms in animals and plants. In plants, its enzymatic generation is still controversial. Evidence points to nitrate reductase being important, but the presence of a nitric oxide synthase-like enzyme is still contested. Regardless, NO has been shown to mediate many developmental stages in plants, and to be involved in a range of physiological responses, from stress management to stomatal aperture closure. Downstream from its generation are alterations of the actions of many cell signaling components, with post-translational modifications of proteins often being key. Here, a collection of papers embraces the differing aspects of NO metabolism in plants. Keywords: nitrate reductase; nitration; nitric oxide; reactive oxygen species; stress responses; S-nitrosation; S-nitrosylation; SNO-reductase; thiol modification 1. Introduction Nitric oxide (NO) is now well acknowledged as an instrumental signaling molecule in both plants and animals [1]. First recognized as important as a signal in the control of vascular tone [2], its role in plants came to prominence in the late 1990s [3–5]. The forty years of research into NO in plants has just been highlighted by a review by Kolbert et al. [6]. In plants, NO has been found to be involved in a wide range of developmental stages and physiological responses. -

Nitro-L-Arginine Methyl Ester (L-NAME) Causes Limb Defects in Mouse Fetuses: Protective Effect of Acute Hyperoxia
0031-3998/03/5401-0069 PEDIATRIC RESEARCH Vol. 54, No. 1, 2003 Copyright © 2003 International Pediatric Research Foundation, Inc. Printed in U.S.A. The Nitric Oxide Synthesis Inhibitor N -Nitro-L-Arginine Methyl Ester (L-NAME) Causes Limb Defects in Mouse Fetuses: Protective Effect of Acute Hyperoxia GIAN MARIO TIBONI, FRANCA GIAMPIETRO, AND CAMILLO DI GIULIO Sezione di Ostetricia e Ginecologia, Dipartimento di Medicina e Scienze dell’Invecchiamento [G.M.T., F.G.], and Sezione di Fisiologia, Dipartimento di Scienze Biomediche [C.d.G.], Facoltà di Medicina e Chirurgia, Università “G. d’Annunzio”, Chieti, Italy. ABSTRACT In the present study the relationship between exposure to the teratogenesis was investigated. To this aim, a group of L-NAME– nitric oxide synthesis inhibitor N -nitro-L-arginine methyl ester treated animals (90 mg/kg s.c. on gestation d 14) were exposed (L-NAME) and the induction of limb defects, with respect to to 98 to 100% O2 for 12 h. L-NAME–treated mice breathing stage specificity and dose dependency, was investigated in the room air served as positive controls. In response to hyperoxia, a mouse. ICR (CD-1) mice were dosed s.c with L-NAME at 50 or significant decrement of L-NAME–induced limb defects was 90 mg/kg on gestation d 12, 13, 14, 15, or 16. A group of animals found. This study characterizes for the first time the teratogenic treated with vehicle on gestation d 14 served as control. Uterine capacity of L-NAME in the mouse. Results obtained with hyper- contents were evaluated for teratogenesis on gestation d 18. -

Influence of Functional Variant of Neuronal Nitric Oxide Synthase on Impulsive Behaviors in Humans
ORIGINAL ARTICLE Influence of Functional Variant of Neuronal Nitric Oxide Synthase on Impulsive Behaviors in Humans Andreas Reif, MD; Christian P. Jacob, MD; Dan Rujescu, MD; Sabine Herterich, PhD; Sebastian Lang, MD; Lise Gutknecht, PhD; Christina G. Baehne, Dipl-Psych; Alexander Strobel, PhD; Christine M. Freitag, MD; Ina Giegling, MD; Marcel Romanos, MD; Annette Hartmann, MD; Michael Rösler, MD; Tobias J. Renner, MD; Andreas J. Fallgatter, MD; Wolfgang Retz, MD; Ann-Christine Ehlis, PhD; Klaus-Peter Lesch, MD Context: Human personality is characterized by sub- Main Outcome Measures: For the association stud- stantial heritability but few functional gene variants have ies, the major outcome criteria were phenotypes rel- been identified. Although rodent data suggest that the evant to impulsivity, namely, the dimensional pheno- neuronal isoform of nitric oxide synthase (NOS-I) modi- type conscientiousness and the categorical phenotypes fies diverse behaviors including aggression, this has not adult ADHD, aggression, and cluster B personality been translated to human studies. disorder. Objectives: To investigate the functionality of an NOS1 Results: A novel functional promoter polymorphism in promoter repeat length variation (NOS1 Ex1f variable NOS1 was associated with traits related to impulsivity, number tandem repeat [VNTR]) and to test whether it including hyperactive and aggressive behaviors. Specifi- is associated with phenotypes relevant to impulsivity. cally, the short repeat variant was more frequent in adult ADHD, cluster B personality disorder, and autoaggres- Design: Molecular biological studies assessed the cel- sive and heteroaggressive behavior. This short variant lular consequences of NOS1 Ex1f VNTR; association came along with decreased transcriptional activity of the studies were conducted to investigate the impact of this genetic variant on impulsivity; imaging genetics was ap- NOS1 exon 1f promoter and alterations in the neuronal plied to determine whether the polymorphism is func- transcriptome including RGS4 and GRIN1. -

Since January 2020 Elsevier Has Created a COVID-19 Resource Centre with Free Information in English and Mandarin on the Novel Coronavirus COVID- 19
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active. Journal Pre-proof Could nitric oxide help to prevent or treat COVID-19? Jan Martel, Yun-Fei Ko, John D. Young, David M. Ojcius PII: S1286-4579(20)30080-0 DOI: https://doi.org/10.1016/j.micinf.2020.05.002 Reference: MICINF 4713 To appear in: Microbes and Infection Received Date: 1 May 2020 Accepted Date: 4 May 2020 Please cite this article as: J. Martel, Y.-F. Ko, J.D. Young, D.M. Ojcius, Could nitric oxide help to prevent or treat COVID-19?, Microbes and Infection, https://doi.org/10.1016/j.micinf.2020.05.002. This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. -

Differential Regulation of Endothelium Behavior by Progesterone and Medroxyprogesterone Acetate
P H CUTINI and others Progestins and vascular function 220:3 179–193 Research Differential regulation of endothelium behavior by progesterone and medroxyprogesterone acetate Pablo H Cutini1,2, Adria´n E Campelo1,2 and Virginia L Massheimer1,2 Correspondence should be addressed 1Ca´ tedra de Bioquı´mica Clı´nica II, Departamento de Biologı´a, Bioquı´mica y Farmacia, Universidad Nacional to V L Massheimer del Sur (UNS), San Juan 670, B8000ICN, Bahı´a Blanca, Argentina Email 2Consejo Nacional de Investigaciones Cientı´ficas y Te´ cnicas (CONICET), Argentina, Buenos Aires, Argentina [email protected] Abstract Medroxyprogesterone acetate (MPA) is a synthetic progestin commonly used in hormone Key Words replacement therapy (HRT). The aim of this research was to study and compare the effect of " cell migration progesterone (Pg) and MPA on the regulation of cellular events associated with vascular " medroxyprogesterone homeostasis and disease. Platelet adhesion to endothelial cells (ECs), nitric oxide (NO) acetate production, and cell migration were studied using murine ECs in vitro exposed to the " nitric oxide progestins. After 7 min of treatment, MPA significantly inhibited NO synthesis with respect " progesterone to control values; meanwhile, Pg markedly increased vasoactive production. In senile ECs, " vascular tissue the stimulatory action of Pg decreases; meanwhile, MPA maintained its ability to inhibit Journal of Endocrinology NO synthesis. The presence of RU486 antagonized the action of each steroid. When ECs were preincubated with PD98059 (MAPK inhibitor) or chelerythrine (protein kinase C (PKC) inhibitor) before Pg or MPA treatment, the former totally suppressed the steroid action, but the PKC antagonist did not affect NO production. -

Progesterone Using ALZET Osmotic Pumps
ALZET® Bibliography References on the Administration of Progesterone Using ALZET Osmotic Pumps Q8558: V. Joseph, et al. Progesterone decreases apnoea and reduces oxidative stress induced by chronic intermittent hypoxia in ovariectomized female rats. Exp Physiol 2020;105(6):1025-1034 Agents: Progesterone Vehicle: Cyclodextrin, 2-ß-Hydroxypropl-; Route: SC; Species: Rat; Pump: 2ML4; Duration: 28 days; ALZET Comments: Dose (4 mg/kg/day); Controls received mp w/ vehicle; animal info (Sprague-Dawley female rats (220-250g/57-70 days old)); post op. care (buprenorphine); Blood pressure measured via tail cuff method;93.3 mmHg - 105.2 mmHg;Progesterone aka prog; dependence; Q6232: S. F. Rosen, et al. T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 2017;37(41):9819-9827 ALZET Comments: Estradiol, 17b-; Progesterone sulfate; SC; Mice; 2002; 14 days; Dose (17b-estradiol : 0.1 mg/kg/d, progesterone sulfate: 0.25 mg/kg/d, 0.1 mg/kg/d estradiol + 0.25 mg/kg/d progesterone); Controls received mp w/ vehicle; animal info (7-12 week old female C57BL/6J mice); replacement therapy (estradiol, ovariectomy); Therapeutic indication. Q6066: D. J. Morris, et al. Glucocorticoids and gut bacteria: "The GALF Hypothesis" in the metagenomic era. Steroids 2017;125(1-13 ALZET Comments: Chenodeoxycholic acid, progesterone, 11b-hydroxy-, corticosterone, deoxy-, corticosterone, 3α,5α-TH-, progesterone, 3α,5α-TH-11β-hydroxy-; SC; Rat; steroidal derivatives of corticosterone; Review presents the role of gut microbial metabolism of endogenous adrenocorticosteroids as a contributing factor in the etiology of essential hypertension. Q6204: S. McIlvride, et al. A progesterone-brown fat axis is involved in regulating fetal growth. -

Nitric Oxide Production, Inhibitory, Antioxidant and Antimycobacterial Activities of the Fruits Extract and Flavonoid Content of Schinus Terebinthifolius
Rev Bras Farmacogn 24(2014): 644-650 Original article Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius Natalia R. Bernardesa, Marlon Heggdorne-Araújoa,b, Isabela F. J. C. Borgesb,c, Fabricio M. Almeidab, Eduardo P. Amaralb, Elena B. Lasunskaiab, Michelle F. Muzitanob,c,*, Daniela B. Oliveiraa,* aLaboratório de Tecnologia de Alimentos, Centro de Ciências e Tecnologias Agropecuárias, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ, Brazil bLaboratório de Biologia do Reconhecer, Centro de Biociências e Biotecnologia, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ, Brazil cLaboratório de Produtos Naturais, Curso de Farmácia, Universidade Federal do Rio de Janeiro, Campus Macaé, Polo Novo Cavaleiro, Instituto Macaé de Metrologia e Tecnologia, Macaé, RJ, Brazil ARTICLE INFO ABSTRACT Article history: The extract of the fruits from Schinus terebinthifolius Raddi, Anacardiaceae, was obtained by Received 28 May 2014 exhaustive extraction with methanol. Its fractions and isolated compounds were collected Accepted 16 October 2014 by fractionation with RP-2 column chromatography. The crude extract, the flavonoid frac- tion and the isolated compound identified as apigenin (1), were investigated regarding its Keywords: inhibitory action of nitric oxide production by LPS-stimulated macrophages, antioxidant Anacardiaceae activity by DPPH and the antimycobacterial activity against Mycobacterium bovis BCG. The Apigenin samples exhibited a significant inhibitory effect on the nitric oxide production (e.g., 1, IC50 Inflammation 19.23 ± 1.64 μg/ml) and also showed antioxidant activity. In addition, S. terebinthifolius sam- Mycobacterium ples inhibited the mycobacterial growth (e.g., 1, IC50 14.53 ± 1.25 μg/ml).