Toxic, Repellent and Antifeedant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plants for Landscapes
Plus 10 Water-Saving Tips for your Garden your for Tips Water-Saving 10 Plus 5 Printed on recycled paper recycled on Printed © 2014 San Diego County Water Authority Water County Diego San 2014 © sdbgarden.org sdbgarden.org thegarden.org tted landscape that looks beautiful and saves water. saves and beautiful looks that landscape tted retrofi or new a for ideas get Landscapes ese gardens are excellent places to to places excellent are gardens Th ese Cajon. El in Garden Conservation Water the and Encinitas Many of the plants in this guide are labeled and on display at the San Diego Botanic Garden in in Garden Botanic Diego San the at display on and labeled are guide this in plants the of Many 0 WaterSmartSD.org Plants for for Plants Nifty agencies member 24 its and hese Nift y 50 plants have been selected because they are attractive, T oft en available in nurseries, non-invasive, easy to maintain, long- term performers, scaled for residential landscapes and, once estab- lished, drought-tolerant. In fact, these plants thrive in San Diego’s semi- arid climate and can help restore regional authenticity to your home. What’s exciting is that authentic also means sustainable. Plants native to Mediterranean climate zones love it here as much as you do. Th ey adapted over thousands of years, and the animal species that depend on them for food and habitat adapted, too. In fact, there are thousands of ground cov- ers, grasses, succulents, perennials, shrubs, vines and trees to choose from. For more information, go to WaterSmartSD.org. -

J. APIC. SCI. Vol. 59 No. 2 2015 DOI: 10.1515/JAS-2015-0028
DOI: 10.1515/JAS-2015-0028 J. APIC. SCI. Vol. 59 No. 2 2015J. APIC. SCI. Vol. 59 No. 2 2015 Original Article FLORAL PHENOLOGY, NECTAR SECRETION DYNAMICS, AND HONEY PRODUCTION POTENTIAL, OF TWO LAVENDER SPECIES (LAVANDULA DENTATA, AND L. PUBESCENS) IN SOUTHWESTERN SAUDI ARABIA Adgaba Nuru* Ahmad A. Al-Ghamdi Yilma T. Tena Awraris G. Shenkut Mohammad J. Ansari Anwer Al-Maktary Engineer Abdullah Bagshan Chair for Bee Research, Department of Plant Protec- tion, College of Food and Agricultural Science, King Saud University Riyadh 11451 Riyadh (P. Box 2460), Saudi Arabia *corresponding author: [email protected] Received 18 August 2015; accepted 07 October 2015 A b s t r a c t The aim of the current study was to determine the floral phenology, nectar secretion dynamics, and honey production potentials of two naturally growing lavender species (L. dentata and L. pubescens), in southwestern Saudi Arabia. In both species, flowering is continuous. This means that, when open flowers on a spike are shaded, new flowers emerge. Such a flowering pattern might be advantageous to the plant to minimise competition for pollinators and promote efficient resource allocation. The flowering periods of the two species overlap. Both species secreted increasing amounts of nectar from early morning to late afternoon. The mean maximum volumes of accumulated nectar from bagged flowers occurred at 15:00 for L. pubescens (0.50 ± 0.24 µL/flower) and at 18:00 for L. dentata (0.68 ± 0.19 µL/flower). The volume of the nectar that became available between two successive measurements (three-h intervals) varied from 0.04 µL/flower to 0.28 µL/flower for L. -
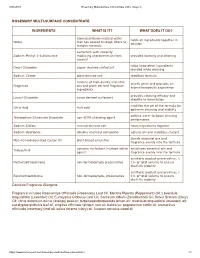
ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? Contains Fragrance Allergens Fragrance Includes Rosm
9/24/2019 Rosemary Multi-Surface Concentrate | Mrs. Meyer's ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? deionized/demineralized water holds all ingredients together in Water that has passed through filters to solution remove minerals surfactant with viscosity Sodium Methyl 2-Sulfolaurate modifying characteristics from provides foaming and cleaning coconut helps keep other ingredients Decyl Glucoside sugar- derived surfactant blended while cleaning Sodium Citrate plant-derived salt stabilizes formula mixture of high quality essential smells great and provides an Fragrance oils and plant-derived fragrance aromatherapeutic experience ingredients provides cleaning efficacy and Lauryl Glucoside sugar derived surfactant stability to formulation modifies the pH of the formula for Citric Acid fruit acid optimum cleaning and stability softens water to boost cleaning Tetrasodium Glutamate Diacetate non-EDTA chelating agent performance Sodium Sulfate mineral-derived salt holds ingredients together Sodium Hydroxide alkaline chemical compound adjusts pH and stabilizes chelant blends essential oils and PEG-40 Hydrogenated Castor Oil plant based emulsifier fragrance evenly into the formula nonionic surfactant (surface active solubilizes essential oils and Trideceth-9 agent) fragrance evenly into the formula synthetic product preservative, < Methylisothiazolinone non-formaldehyde preservative 1% of total volume to ensure shelf life stability synthetic product preservative, < Benzisothiazolinone non-formaldehyde, preservative -

Études Botaniques, Chimiques Et Thérapeutiques Maud Belmont
Lavandula angustifolia M., Lavandula latifolia M., Lavandula x intermedia E. : études botaniques, chimiques et thérapeutiques Maud Belmont To cite this version: Maud Belmont. Lavandula angustifolia M., Lavandula latifolia M., Lavandula x intermedia E. : études botaniques, chimiques et thérapeutiques. Sciences pharmaceutiques. 2013. dumas-00858644 HAL Id: dumas-00858644 https://dumas.ccsd.cnrs.fr/dumas-00858644 Submitted on 5 Sep 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il n’a pas été réévalué depuis la date de soutenance. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D’autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact au SICD1 de Grenoble : [email protected] LIENS LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de la Propriété Intellectuelle. articles L 335.2- L 335.10 http://www.cfcopies.com/V2/leg/leg_droi.php http://www.culture.gouv.fr/culture/infos-pratiques/droits/protection.htm UNIVERSITÉ JOSEPH FOURIER FACULTÉ DE PHARMACIE DE GRENOBLE Année 2013 Lavandula angustifolia M., Lavandula latifolia M., Lavandula x intermedia E.: ÉTUDES BOTANIQUES, CHIMIQUES ET THÉRAPEUTIQUES. -

A Weevil of the Genus Caulophilus in Dominican Amber (Coleoptera: Curculionidae)
POLSKIE P I S M O ENTOMOLOGICZNE P O L I S H JOURNAL OF ENTOMOLOGY VOL. 75: 101-104 Bydgoszcz 2006 A weevil of the genus Caulophilus in Dominican amber (Coleoptera: Curculionidae) STEVEN R. D AVIS , MICHAEL S. E NGEL Division of Entomology, Natural History Museum, and Department of Ecology & Evolutionary Biology, 1345 Jayhawk Boulevard, Dyche Hall, University of Kansas, Lawrence, Kansas 66045-7163, United States ABSTRACT. The remains of a new species of cossonine weevil (Cossoninae: Dryotribini) are described and figured from Early Miocene (Burdigalian) amber from the Dominican Republic. Caulophilus ashei sp. n. is distinguished from other species in the genus. KEY WORDS: Polyphaga, Phytophaga, Tertiary, Curculionoidea, palaeontology, taxonomy. INTRODUCTION Weevils of the cossonine genus Caulophilus WOLLASTON are familiar members of the tribe Dryotribini. The common European species, Caulophilus oryzae (GYLLENHAL ) is widely known as the broadnose grain weevil and a frequent pest in agricultural systems. Adult females lay small, white eggs in soft or damaged grains (frequently damaged by the activity of other grain insects) which then hatch within a few days. The larvae feed on the softer portions of the grain before pupating and finally eclosing to an adult. Herein we provide the description of the first fossil Caulophilus , the first fossil of the tribe Dryotribini, and the first cossonine in Dominican amber. Today the genus Caulophilus consists of 17 described species distributed in South and North America, Puerto Rico, Jamaica, Cuba, Hawaii, Seychelles, Madeira, and Europe, although some have become recently adventive in other regions (e.g., New Zealand). With the discovery of the species described herein the genus can now be documented from paleofauna of Hispaniola and is at least 19 million years old. -

Coleoptera) (Excluding Anthribidae
A FAUNAL SURVEY AND ZOOGEOGRAPHIC ANALYSIS OF THE CURCULIONOIDEA (COLEOPTERA) (EXCLUDING ANTHRIBIDAE, PLATPODINAE. AND SCOLYTINAE) OF THE LOWER RIO GRANDE VALLEY OF TEXAS A Thesis TAMI ANNE CARLOW Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 1997 Major Subject; Entomology A FAUNAL SURVEY AND ZOOGEOGRAPHIC ANALYSIS OF THE CURCVLIONOIDEA (COLEOPTERA) (EXCLUDING ANTHRIBIDAE, PLATYPODINAE. AND SCOLYTINAE) OF THE LOWER RIO GRANDE VALLEY OF TEXAS A Thesis by TAMI ANNE CARLOW Submitted to Texas AgcM University in partial fulltllment of the requirements for the degree of MASTER OF SCIENCE Approved as to style and content by: Horace R. Burke (Chair of Committee) James B. Woolley ay, Frisbie (Member) (Head of Department) Gilbert L. Schroeter (Member) August 1997 Major Subject: Entomology A Faunal Survey and Zoogeographic Analysis of the Curculionoidea (Coleoptera) (Excluding Anthribidae, Platypodinae, and Scolytinae) of the Lower Rio Grande Valley of Texas. (August 1997) Tami Anne Carlow. B.S. , Cornell University Chair of Advisory Committee: Dr. Horace R. Burke An annotated list of the Curculionoidea (Coleoptem) (excluding Anthribidae, Platypodinae, and Scolytinae) is presented for the Lower Rio Grande Valley (LRGV) of Texas. The list includes species that occur in Cameron, Hidalgo, Starr, and Wigacy counties. Each of the 23S species in 97 genera is tteated according to its geographical range. Lower Rio Grande distribution, seasonal activity, plant associations, and biology. The taxonomic atTangement follows O' Brien &, Wibmer (I og2). A table of the species occuning in patxicular areas of the Lower Rio Grande Valley, such as the Boca Chica Beach area, the Sabal Palm Grove Sanctuary, Bentsen-Rio Grande State Park, and the Falcon Dam area is included. -

The Curculionoidea of the Maltese Islands (Central Mediterranean) (Coleoptera)
BULLETIN OF THE ENTOMOLOGICAL SOCIETY OF MALTA (2010) Vol. 3 : 55-143 The Curculionoidea of the Maltese Islands (Central Mediterranean) (Coleoptera) David MIFSUD1 & Enzo COLONNELLI2 ABSTRACT. The Curculionoidea of the families Anthribidae, Rhynchitidae, Apionidae, Nanophyidae, Brachyceridae, Curculionidae, Erirhinidae, Raymondionymidae, Dryophthoridae and Scolytidae from the Maltese islands are reviewed. A total of 182 species are included, of which the following 51 species represent new records for this archipelago: Araecerus fasciculatus and Noxius curtirostris in Anthribidae; Protapion interjectum and Taeniapion rufulum in Apionidae; Corimalia centromaculata and C. tamarisci in Nanophyidae; Amaurorhinus bewickianus, A. sp. nr. paganettii, Brachypera fallax, B. lunata, B. zoilus, Ceutorhynchus leprieuri, Charagmus gressorius, Coniatus tamarisci, Coniocleonus pseudobliquus, Conorhynchus brevirostris, Cosmobaris alboseriata, C. scolopacea, Derelomus chamaeropis, Echinodera sp. nr. variegata, Hypera sp. nr. tenuirostris, Hypurus bertrandi, Larinus scolymi, Leptolepurus meridionalis, Limobius mixtus, Lixus brevirostris, L. punctiventris, L. vilis, Naupactus cervinus, Otiorhynchus armatus, O. liguricus, Rhamphus oxyacanthae, Rhinusa antirrhini, R. herbarum, R. moroderi, Sharpia rubida, Sibinia femoralis, Smicronyx albosquamosus, S. brevicornis, S. rufipennis, Stenocarus ruficornis, Styphloderes exsculptus, Trichosirocalus centrimacula, Tychius argentatus, T. bicolor, T. pauperculus and T. pusillus in Curculionidae; Sitophilus zeamais and -

PDF 25 Kb) Approved the Final Manuscript
Bajda et al. BMC Genomics (2015) 16:974 DOI 10.1186/s12864-015-2157-1 RESEARCH ARTICLE Open Access Transcriptome profiling of a spirodiclofen susceptible and resistant strain of the European red mite Panonychus ulmi using strand-specific RNA-seq Sabina Bajda1†, Wannes Dermauw2*† , Robert Greenhalgh3, Ralf Nauen4, Luc Tirry2, Richard M. Clark3,5 and Thomas Van Leeuwen1,2* Abstract Background: The European red mite, Panonychus ulmi, is among the most important mite pests in fruit orchards, where it is controlled primarily by acaricide application. However, the species rapidly develops pesticide resistance, and the elucidation of resistance mechanisms for P. ulmi has not kept pace with insects or with the closely related spider mite Tetranychus urticae. The main reason for this lack of knowledge has been the absence of genomic resources needed to investigate the molecular biology of resistance mechanisms. Results: Here, we provide a comprehensive strand-specific RNA-seq based transcriptome resource for P. ulmi derived from strains susceptible and resistant to the widely used acaricide spirodiclofen. From a de novo assembly of the P. ulmi transcriptome, we manually annotated detoxification enzyme families, target-sites of commonly used acaricides, and horizontally transferred genes implicated in plant-mite interactions and pesticide resistance. In a comparative analysis that incorporated sequences available for Panonychus citri, T. urticae, and insects, we identified radiations for detoxification gene families following the divergence of Panonychus and Tetranychus genera. Finally, we used the replicated RNA-seq data from the spirodiclofen susceptible and resistant strains to describe gene expression changes associated with resistance. A cytochrome P450 monooxygenase, as well as multiple carboxylcholinesterases, were differentially expressed between the susceptible and resistant strains, and provide a molecular entry point for understanding resistance to spirodiclofen, widely used to control P. -

ANNUAL REPORT 2020 Plant Protection & Conservation Programs
Oregon Department of Agriculture Plant Protection & Conservation Programs ANNUAL REPORT 2020 www.oregon.gov/ODA Plant Protection & Conservation Programs Phone: 503-986-4636 Website: www.oregon.gov/ODA Find this report online: https://oda.direct/PlantAnnualReport Publication date: March 2021 Table Tableof Contents of Contents ADMINISTRATION—4 Director’s View . 4 Retirements: . 6 Plant Protection and Conservation Programs Staff . 9 NURSERY AND CHRISTMAS TREE—10 What Do We Do? . 10 Christmas Tree Shipping Season Summary . 16 Personnel Updates . .11 Program Overview . 16 2020: A Year of Challenge . .11 New Rule . 16 Hawaii . 17 COVID Response . 12 Mexico . 17 Funding Sources . 13 Nursery Research Assessment Fund . 14 IPPM-Nursery Surveys . 17 Phytophthora ramorum Nursery Program . 14 National Traceback Investigation: Ralstonia in Oregon Nurseries . 18 Western Horticultural Inspection Society (WHIS) Annual Meeting . 19 HEMP—20 2020 Program Highlights . 20 2020 Hemp Inspection Annual Report . 21 2020 Hemp Rule-making . 21 Table 1: ODA Hemp Violations . 23 Hemp Testing . .24 INSECT PEST PREVENTION & MANAGEMENT—25 A Year of Personnel Changes-Retirements-Promotions High-Tech Sites Survey . .33 . 26 Early Detection and Rapid Response for Exotic Bark Retirements . 27 and Ambrosia Beetles . 33 My Unexpected Career With ODA . .28 Xyleborus monographus Early Detection and Rapid Response (EDRR) Trapping . 34 2020 Program Notes . .29 Outreach and Education . 29 Granulate Ambrosia Beetle and Other Wood Boring Insects Associated with Creosoting Plants . 34 New Detections . .29 Japanese Beetle Program . .29 Apple Maggot Program . .35 Exotic Fruit Fly Survey . .35 2018 Program Highlights . .29 Japanese Beetle Eradication . .30 Grasshopper and Mormon Cricket Program . .35 Grasshopper Outbreak Response – Harney County . -

Predation of the Chinch Bug, Blissus Occiduus Barber (Hemiptera: Blissidae) by Geocoris Uliginosus (Say) (Hemiptera: Lygaeidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications: Department of Entomology Entomology, Department of 2008 Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae) J. D. Carstens University of Nebraska-Lincoln Frederick P. Baxendale University of Nebraska-Lincoln, [email protected] Tiffany Heng-Moss University of Nebraska-Lincoln, [email protected] Robert J. Wright University of Nebraska, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/entomologyfacpub Part of the Entomology Commons Carstens, J. D.; Baxendale, Frederick P.; Heng-Moss, Tiffany; and Wright, Robert J., "Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae)" (2008). Faculty Publications: Department of Entomology. 157. https://digitalcommons.unl.edu/entomologyfacpub/157 This Article is brought to you for free and open access by the Entomology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications: Department of Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. JOURNAL OF THE KANSAS ENTOMOLOGICAL SOCIETY 81(4), 2008, pp. 328–338 Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae) J. D. CARSTENS,F.P.BAXENDALE,T.M.HENG-MOSS, AND R. J. WRIGHT Department of Entomology, University of Nebraska-Lincoln, Lincoln, NE 68583 ABSTRACT: Big-eyed bugs have been well documented as predators on a diverse group of arthropod prey in turfgrasses; however, little is known about the big-eyed bug species associated with buffalograss, or their feeding habits relative to the western chinch bug, Blissus occiduus Barber. -

Sharp's at Waterford Farm Your Neighborhood Farm Ask Us How To
Lemongrass – Essential for Thai Sharp’s at Waterford Herbs List cooking Farm Anise - Hyssop Lovage (Levistcum officinale) Farming in Howard County Basil Marjoram (Origanum majorana) since 1903 African Blue Amethyst Improved Purple Sweet Eleonora Zaatar, a hint of thyme, oregano & 4003 Jennings Chapel Rd. Elidia - Compact; container basil marjoram Brookeville, MD 20833 Genovese Golden - ornamental mostly Holy - Sacred Red and Green Tel: (410) 489-2572 Mint (Mentha sp.) Italian Large Leaf Chocolate Peppermint Lemon – Mrs. Burns www.sharpfarm.com Lemon Mint Mountain Mint Lettuce Leaf – Napoletano email: Peppermint Pineapple Mint Lime [email protected] Spearmint Sweet Thai Dark Opal Oregano (Origanum sp.) Red Rubin Greek Rutgers Devotion Zaatar ( a hint of thyme, oregano, & marjoram) Oreganum Syriaca) Borage: the herb of gladness Hot and Spicy - real tang, our favorite for adding to beans Catnip (Nepeta)- feline friends treat Parsley (Petroselinum crispum) Calendula, Neon Plain leaf (Italian or flat) Curly – double or triple Chamomile (German) Organic curled parsley (Bodegold) Italian Dark Green – Giant of Italy – huge leaves Your Neighborhood Chervil (Anthricus cerefolium) ‘crispum’ Vertissimo Farm Rosemary (Rosmarinus) Arp Chives (Allium) Hill Hardy Med Leaf (Purly) Ask Us How to Garden Salem Large leaf (staro) Sage (Salvia offincinalis) Helpful Hints: We pride ourselves Cilantro (Coriandrum sativium) Garden - Extrakta on knowing how to vegetable and herb Cruiser – more upright – great for Pineapple garden. Please ask if you need bunching – 50 days Savory Winter information on how to. Yields? Cutting Celery (Apium graveolens) Sorrel, French Spacing between plants? Staking? aka leaf celery When you plant, space your harvest Stevia (Stevia rebaudiana) by using varieties of different maturity Dill (Anethum graveolens): Nature’s natural sweetener dates. -

Behavioral Response of Panonychus Citri (Mcgregor) (Acari: Tetranychidae) to Synthetic Chemicals and Oils
Behavioral response of Panonychus citri (McGregor) (Acari: Tetranychidae) to synthetic chemicals and oils Muhammad Asif Qayyoum1,2,*, Zi-Wei Song1,*, Bao-Xin Zhang1, Dun-Song Li1 and Bilal Saeed Khan3 1 Guangdong Provincial Key Laboratory of High Technology for Plant Protection/Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou City, Guangdong, China 2 Department of Plant Protection, Ghazi University, Dera Ghazi Khan, Dera Ghazi Khan, Punjab, Pakistan 3 Department of Entomology, University of Agriculture Faisalabad, Faisalabad, Punjab, Pakistan * These authors contributed equally to this work. ABSTRACT Background: Panonychus citri (McGregor) (Acari: Tetranychidae) population outbreaks after the citrus plantation’s chemical application is a common observation. Dispersal behavior is an essential tool to understand the secondary outbreak of P. citri population. Therefore, in the current study, the dispersal activity of P. citri was observed on the leaf surfaces of Citrus reticulata (Rutaceae) treated with SYP-9625, abamectin, vegetable oil, and EnSpray 99. Method: Mites were released on the first (apex) leaf of the plant (adaxial surface) and data were recorded after 24 h. The treated, untreated, and half-treated data were analyzed by combining the leaf surfaces (adaxial right, adaxial left, abaxial right, and abaxial left). All experiments were performed in open-air environmental conditions. Results: The maximum number of mites was captured on the un-treated or half-treated surfaces due to chemicals repellency. Chemical bioassays of the free-choice test showed that all treatments significantly increased the mortality of Submitted 10 September 2020 P. citri depending on application method and concentration. A significant number 13 January 2021 Accepted of mites repelled away from treated surfaces and within treated surfaces except Published 5 April 2021 adaxial left and abaxial right surfaces at LC30.