Interannual Variation in the Diets of Piliocolobus Badius Badius from the Taï Forest of Cote D'ivoire THESIS Presented In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physical, Mechanical, and Other Properties Of
ARC: 634.9 TA/OST 73-24 C559a PHYSICAL, MECHANICAL, AND OTHER PROPERTIES OF SELECTED SECONDARY SPECIES in Surinam, Peru, Colombia, Nigeria, Gabon, Philippines, and Malaysia FPL-AID-PASA TA(Aj)2-73 (Species Properties) * PHYSICAL, MECHANICAL, AND OTHER PROPERTIES OF SELECTED SECONDARY SPECIES LOCATED IN SURINAM, PERU, COLOMBIA, NIGERIA, GABON, PHILIPPINES, AND MALAYSIA MARTIN CHUDNOFF, Forest Products Technologist Forest Products Laboratory Forest Service, U.S. Department of Agriculture Madison, Wisconsin 53705 November 1973 Prepared for AGENCY FOR INTERNATIONAL DEVELOPMENT U.S. Department of State Washington, DC 20523 ARC No. 634.9 - C 559a INTRODUCTION This report is a partial response to a Participating Agency Service Agreement between the Agency for Inter national Development and the USDA, Forest Service (PASA Control No. TA(AJ)2-73) and concerns a study of the factors influencing the utilization of the tropical forest resource. The purpose of this portion of the PASA obligation is to present previously published information on the tree and wood characteristics of selected secondary species growing m seven tropical countries. The format is concise and follows the outline developed for the second edition of the "Handbook of Hardwoods" published by HMSO, London. Species selected for review are well known in the source countries, but make up a very small component, if any, of their export trade. The reasons why these species play a secondary role in the timber harvest are discussed in the other accompanying PASA reports. ii INDEX Pages SURINAM 1-11 Audira spp. Eperu falcata Eschweilera spp. Micropholis guyanensis Nectandra spp. Ocotea spp. Parinari campestris Parinari excelsa Pouteria engleri Protium spp. -

Activity and Ranging Patterns of Guerezas in the Kakamega Forest: Intergroup Variation and Implications for Intragroup Feeding Competition
P1: Vendor International Journal of Primatology [ijop] PP095-296772 June 6, 2001 11:50 Style file version Nov. 19th, 1999 International Journal of Primatology, Vol. 22, No. 4, 2001 Activity and Ranging Patterns of Guerezas in the Kakamega Forest: Intergroup Variation and Implications for Intragroup Feeding Competition Peter J. Fashing1,2 Received November 29, 1999; revision July 13, 2000; accepted August 10, 2000 From March 1997 to February 1998, I investigated the activity patterns of 2 groups and the ranging patterns of 5 groups of eastern black-and-white colobus (Colobus guereza), aka guerezas, in the Kakamega Forest, Kenya. Guerezas at Kakamega spent more of their time resting than any other pop- ulation of colobine monkeys studied to date. In addition, I recorded not one instance of intragroup aggression in 16,710 activity scan samples, providing preliminary evidence that intragroup contest competition may be rare or ab- sent among guerezas at Kakamega. Mean daily path lengths ranged from 450 to 734 m, and home range area ranged from 12 to 20 ha, though home range area may have been underestimated for several of the study groups. Home range overlap was extensive with 49–83% of each group’s range overlapped by the ranges of other groups. Despite the high level of home range overlap, the frequently entered areas (quadrats entered on 30% of a group’s total study days) of any one group were not frequently entered by any other study group. Mean daily path length is not significantly correlated with levels of availability or consumption of any plant part item. -

Method to Estimate Dry-Kiln Schedules and Species Groupings: Tropical and Temperate Hardwoods
United States Department of Agriculture Method to Estimate Forest Service Forest Dry-Kiln Schedules Products Laboratory Research and Species Groupings Paper FPL–RP–548 Tropical and Temperate Hardwoods William T. Simpson Abstract Contents Dry-kiln schedules have been developed for many wood Page species. However, one problem is that many, especially tropical species, have no recommended schedule. Another Introduction................................................................1 problem in drying tropical species is the lack of a way to Estimation of Kiln Schedules.........................................1 group them when it is impractical to fill a kiln with a single Background .............................................................1 species. This report investigates the possibility of estimating kiln schedules and grouping species for drying using basic Related Research...................................................1 specific gravity as the primary variable for prediction and grouping. In this study, kiln schedules were estimated by Current Kiln Schedules ..........................................1 establishing least squares relationships between schedule Method of Schedule Estimation...................................2 parameters and basic specific gravity. These relationships were then applied to estimate schedules for 3,237 species Estimation of Initial Conditions ..............................2 from Africa, Asia and Oceana, and Latin America. Nine drying groups were established, based on intervals of specific Estimation -

Plants for Tropical Subsistence Farms
SELECTING THE BEST PLANTS FOR THE TROPICAL SUBSISTENCE FARM By Dr. F. W. Martin. Published in parts, 1989 and 1994; Revised 1998 and 2007 by ECHO Staff Dedication: This document is dedicated to the memory of Scott Sherman who worked as ECHO's Assistant Director until his death in January 1996. He spent countless hours corresponding with hundreds of missionaries and national workers around the world, answering technical questions and helping them select new and useful plants to evaluate. Scott took special joy in this work because he Photo by ECHO Staff knew the God who had created these plants--to be a blessing to all the nations. WHAT’S INSIDE: TABLE OF CONTENTS HOW TO FIND THE BEST PLANTS… Plants for Feeding Animals Grasses DESCRIPTIONS OF USEFUL PLANTS Legumes Plants for Food Other Feed Plants Staple Food Crops Plants for Supplemental Human Needs Cereal and Non-Leguminous Grain Fibers Pulses (Leguminous Grains) Thatching/Weaving and Clothes Roots and Tubers Timber and Fuel Woods Vegetable Crops Plants for the Farm Itself Leguminous Vegetables Crops to Conserve or Improve the Soil Non-Leguminous Fruit Vegetables Nitrogen-Fixing Trees Leafy Vegetables Miners of Deep (in Soil) Minerals Miscellaneous Vegetables Manure Crops Fruits and Nut Crops Borders Against Erosion Basic Survival Fruits Mulch High Value Fruits Cover Crops Outstanding Nuts Crops to Modify the Climate Specialty Food Crops Windbreaks Sugar, Starch, and Oil Plants for Shade Beverages, Spices and Condiment Herbs Other Special-Purpose Plants Plants for Medicinal Purposes Living Fences Copyright © ECHO 2007. All rights reserved. This document may be reproduced for training purposes if Plants for Alley Cropping distributed free of charge or at cost and credit is given to ECHO. -

Biodiversity and Floristic Composition of Medicinal Plants of Darbhanga, Bihar, India
Int.J.Curr.Microbiol.App.Sci (2015) 4(12): 263-283 ISSN: 2319-7706 Volume 4 Number 12 (2015) pp. 263-283 http://www.ijcmas.com Original Research Article Biodiversity and Floristic Composition of Medicinal Plants of Darbhanga, Bihar, India Jyoti Jyotsna1 and Baidyanath Kumar2* 1Research Scholar, PG. Department of Biotechnology, L. N. Mithila University, Darbhanga, Bihar, India 2Visiting Professor, Department of Biotechnology, Patna Science College, Patna, Patna University, Bihar, India *Corresponding author A B S T R A C T The biodiversity, floristic composition and structure of medicinal plants in the eighteen blocks of Darbhanga were studied. A total of 101 plant species belonging to 32 families, 71 genera and 5 life forms were recorded. Fabaceae, Moraceae, Meliaceae and Apocyanaceae were the overall diverse families (in terms of species richness) of the adult species, contributing 44.5% of all the species in the study. K e y w o r d s Trees were the most dominant life form (46.5%) followed by lianas (14.8%), herbs (9.9%), epiphytes (7.9%), shrubs (2.9 %) and the others (3.7%). Species richness Biodiversity, among all life forms was highest in the DB (90.5%). Fabaceae, Moraceae and Floristic Meliaceae and Meliaceae were the most diverse families distributed in all the composition eighteen blocks of Darbhang The trees in all the forest types studied were generally and structure, tall. The difference in height of tree species could be partly explained by Medicinal degradation in the form of logging of tall and big trees which has undoubtedly plants and affected the vertical structure. -

Diversification of Tree Crops: Domestication of Companion Crops for Poverty Reduction and Environmental Services
Expl Agric. (2001), volume 37, pp. 279±296 Printed in Great Britain Copyright # 2001 Cambridge University Press REVIEW PAPER DIVERSIFICATION OF TREE CROPS: DOMESTICATION OF COMPANION CROPS FOR POVERTY REDUCTION AND ENVIRONMENTAL SERVICES By R. R. B. LEAKEY{ and Z. TCHOUNDJEU{ {Centre for Ecology and Hydrology, Bush Estate, Penicuik, Midlothian, EH26 0QB, Scotland, UK and {International Centre for Research in Agroforestry, PO Box 2067, YaoundeÂ, Cameroon (Accepted 19 January 2001) SUMMARY New initiatives in agroforestry are seeking to integrate indigenous trees, whose products have traditionally been gathered from natural forests, into tropical farming systems such as cacao farms. This is being done to provide from farms, marketable timber and non-timber forest products that will enhance rural livelihoods by generating cash for resource-poor rural and peri-urban households. There are many potential candidate species for domestication that have commercial potential in local, regional or even international markets. Little or no formal research has been carried out on many of these hitherto wild species to assess potential for genetic improvement, reproductive biology or suitability for cultivation. With the participation of subsistence farmers a number of projects to bring candidate species into cultivation are in progress, however. This paper describes some tree domestication activities being carried out in southern Cameroon, especially with Irvingia gabonensis (bush mango; dika nut) and Dacryodes edulis (African plum; safoutier). As part of this, fruits and kernels from 300 D. edulis and 150 I. gabonensis trees in six villages of Cameroon and Nigeria have been quantitatively characterized for 11 traits to determine combinations de®ning multi-trait ideotypes for a genetic selection programme. -

The Woods of Liberia
THE WOODS OF LIBERIA October 1959 No. 2159 UNITED STATES DEPARTMENT OF AGRICULTURE FOREST PRODUCTS LABORATORY FOREST SERVICE MADISON 5, WISCONSIN In Cooperation with the University of Wisconsin THE WOODS OF LIBERIA1 By JEANNETTE M. KRYN, Botanist and E. W. FOBES, Forester Forest Products Laboratory,2 Forest Service U. S. Department of Agriculture - - - - Introduction The forests of Liberia represent a valuable resource to that country-- especially so because they are renewable. Under good management, these forests will continue to supply mankind with products long after mined resources are exhausted. The vast treeless areas elsewhere in Africa give added emphasis to the economic significance of the forests of Liberia and its neighboring countries in West Africa. The mature forests of Liberia are composed entirely of broadleaf or hardwood tree species. These forests probably covered more than 90 percent of the country in the past, but only about one-third is now covered with them. Another one-third is covered with young forests or reproduction referred to as low bush. The mature, or "high," forests are typical of tropical evergreen or rain forests where rainfall exceeds 60 inches per year without pro longed dry periods. Certain species of trees in these forests, such as the cotton tree, are deciduous even when growing in the coastal area of heaviest rainfall, which averages about 190 inches per year. Deciduous species become more prevalent as the rainfall decreases in the interior, where the driest areas average about 70 inches per year. 1The information here reported was prepared in cooperation with the International Cooperation Administration. 2 Maintained at Madison, Wis., in cooperation with the University of Wisconsin. -

Hunting, Law Enforcement, and African Primate Conservation
Research Note Hunting, Law Enforcement, and African Primate Conservation PAUL K. N’GORAN,∗†‡§ CHRISTOPHE BOESCH,∗†‡ ROGER MUNDRY,∗ ELIEZER K. N’GORAN,∗∗ ILKA HERBINGER,‡ FABRICE A. YAPI,†† AND HJALMAR S. KUHL¨ ∗‡‡ ∗Department of Primatology, Max Planck Institute for Evolutionary Anthropology, Deutscher Platz 6, 04103 Leipzig, Germany †Centre Suisse de Recherches Scientifiques en Cote-d’Ivoire,ˆ 01 BP 1303 Abidjan 01, Coteˆ d’Ivoire ‡Wild Chimpanzee Foundation s/c CSRS 01 BP 1303 Abidjan 01, Coteˆ d’Ivoire §UFR Sciences de la Nature, Universite´ d’Abobo-Adjame,´ 02 BP 801 Abidjan 02, Coteˆ d’Ivoire ∗∗UFR Biosciences, Universite´ de Cocody, 01 BP V34 Abidjan 01, Coteˆ d’Ivoire ††Office Ivoirien des Parcs et Reserves,´ Direction de Zone Sud-Ouest, BP 1342 Soubre,´ Coteˆ d’Ivoire Abstract: Primates are regularly hunted for bushmeat in tropical forests, and systematic ecological monitor- ing can help determine the effect hunting has on these and other hunted species. Monitoring can also be used to inform law enforcement and managers of where hunting is concentrated. We evaluated the effects of law enforcement informed by monitoring data on density and spatial distribution of 8 monkey species in Ta¨ıNa- tional Park, Coteˆ d’Ivoire. We conducted intensive surveys of monkeys and looked for signs of human activity throughout the park. We also gathered information on the activities of law-enforcement personnel related to hunting and evaluated the relative effects of hunting, forest cover and proximity to rivers, and conservation effort on primate distribution and density. The effects of hunting on monkeys varied among species. Red colobus monkeys (Procolobus badius) were most affected and Campbell’s monkeys (Cercopithecus campbelli) were least affected by hunting. -

Habitat Utilization and Feeding Biology of Himalayan Grey Langur
动 物 学 研 究 2010,Apr. 31(2):177−188 CN 53-1040/Q ISSN 0254-5853 Zoological Research DOI:10.3724/SP.J.1141.2010.02177 Habitat Utilization and Feeding Biology of Himalayan Grey Langur (Semnopithecus entellus ajex) in Machiara National Park, Azad Jammu and Kashmir, Pakistan Riaz Aziz Minhas1,*, Khawaja Basharat Ahmed2, Muhammad Siddique Awan2, Naeem Iftikhar Dar3 (1. World Wide Fund for Nature-Pakistan (WWF-Pakistan) AJK Office Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan; 2. Department ofZoology, University of Azad Jammu and Kashmir Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan; 3. Department of Wildlife and Fisheries, Government of Azad Jammu and Kashmir, Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan) Abstract: Habitat utilization and feeding biology of Himalayan Grey Langur (Semnopithecus entellus ajex) were studied from April, 2006 to April, 2007 in Machiara National Park, Azad Jammu and Kashmir, Pakistan. The results showed that in the winter season the most preferred habitat of the langurs was the moist temperate coniferous forests interspersed with deciduous trees, while in the summer season they preferred to migrate into the subalpine scrub forests at higher altitudes. Langurs were folivorous in feeding habit, recorded as consuming more than 49 plant species (27 in summer and 22 in winter) in the study area. The mature leaves (36.12%) were preferred over the young leaves (27.27%) while other food components comprised of fruits (17.00%), roots (9.45%), barks (6.69%), flowers (2.19%) and stems (1.28%) of various plant species. Key words: Himalayan Grey Langur; Habitat; Food biology; Machiara National Park 喜马拉雅灰叶猴栖息地利用和食性生物学 Riaz Aziz Minhas1,*, Khawaja Basharat Ahmed2, Muhammad Siddique Awan2, Naeem Iftikhar Dar3 (1. -

The Role of Exposure in Conservation
Behavioral Application in Wildlife Photography: Developing a Foundation in Ecological and Behavioral Characteristics of the Zanzibar Red Colobus Monkey (Procolobus kirkii) as it Applies to the Development Exhibition Photography Matthew Jorgensen April 29, 2009 SIT: Zanzibar – Coastal Ecology and Natural Resource Management Spring 2009 Advisor: Kim Howell – UDSM Academic Director: Helen Peeks Table of Contents Acknowledgements – 3 Abstract – 4 Introduction – 4-15 • 4 - The Role of Exposure in Conservation • 5 - The Zanzibar Red Colobus (Piliocolobus kirkii) as a Conservation Symbol • 6 - Colobine Physiology and Natural History • 8 - Colobine Behavior • 8 - Physical Display (Visual Communication) • 11 - Vocal Communication • 13 - Olfactory and Tactile Communication • 14 - The Importance of Behavioral Knowledge Study Area - 15 Methodology - 15 Results - 16 Discussion – 17-30 • 17 - Success of the Exhibition • 18 - Individual Image Assessment • 28 - Final Exhibition Assessment • 29 - Behavioral Foundation and Photography Conclusion - 30 Evaluation - 31 Bibliography - 32 Appendices - 33 2 To all those who helped me along the way, I am forever in your debt. To Helen Peeks and Said Hamad Omar for a semester of advice, and for trying to make my dreams possible (despite the insurmountable odds). Ali Ali Mwinyi, for making my planning at Jozani as simple as possible, I thank you. I would like to thank Bi Ashura, for getting me settled at Jozani and ensuring my comfort during studies. Finally, I am thankful to the rangers and staff of Jozani for welcoming me into the park, for their encouragement and support of my project. To Kim Howell, for agreeing to support a project outside his area of expertise, I am eternally grateful. -
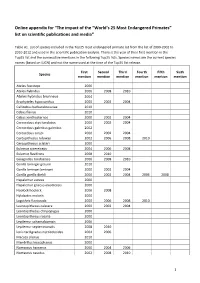
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 7-30-2008 Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St. Louis Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Sweeney, Patrick Wayne, "Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)" (2008). Dissertations. 539. https://irl.umsl.edu/dissertation/539 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. SYSTEMATICS AND FLORAL EVOLUTION IN THE PLANT GENUS GARCINIA (CLUSIACEAE) by PATRICK WAYNE SWEENEY M.S. Botany, University of Georgia, 1999 B.S. Biology, Georgia Southern University, 1994 A DISSERTATION Submitted to the Graduate School of the UNIVERSITY OF MISSOURI- ST. LOUIS In partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in BIOLOGY with an emphasis in Plant Systematics November, 2007 Advisory Committee Elizabeth A. Kellogg, Ph.D. Peter F. Stevens, Ph.D. P. Mick Richardson, Ph.D. Barbara A. Schaal, Ph.D. © Copyright 2007 by Patrick Wayne Sweeney All Rights Reserved Sweeney, Patrick, 2007, UMSL, p. 2 Dissertation Abstract The pantropical genus Garcinia (Clusiaceae), a group comprised of more than 250 species of dioecious trees and shrubs, is a common component of lowland tropical forests and is best known by the highly prized fruit of mangosteen (G. mangostana L.). The genus exhibits as extreme a diversity of floral form as is found anywhere in angiosperms and there are many unresolved taxonomic issues surrounding the genus.