Contraception
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Njm Vol 15.4 Final Correction
REVIEW ARTICLE Current Concepts in Contraception A. M. Abasiattai MBBCH, FWACS Department of Obstetrics/Gynaecology, University of Uyo Teaching Hospital, Uyo, Nigeria ABSTRACT Background: Worldwide, contraceptive use has expensive contraceptives that are easier to deliver and increased substantially over the past two decades. The cause fewer side effects than currently available increased demand for wider choices of contraceptive options4. This article thus focuses on recent methods has resulted in extensive research and developments of existing contraceptive methods and rigorous clinical trials. This has led to improvements on also reviews recently developed methods currently in existing contraceptive methods and also the use worldwide. development of several new, more effective and acceptable methods with fewer side effects. Thus, this HORMONAL METHODS article presents a review of existing literature on recent Combined Oral Contraceptives (COCs) developments on existing contraceptive methods. It These are tablets that contain a combination of also reviews recently developed contraceptive methods oestrogen and progestin and are taken daily. Though currently in use worldwide. they act primarily by suppressing ovulation, they also Methods: Relevant literature was reviewed using thicken cervical mucus thus making it impervious to manual library search, electronic sources such as CD- sperm and alter the uterine endometrium 5,6. They are ROMS and internet articles. safe, very effective when used consistently and Conclusion: More effective methods of contraception accurately with a failure rate of 0.1 in 100 pregnancies in which are generally safer and easier to administer are the first year of use 6. Currently, the development of low increasingly being developed. Hopefully, as they dose formulations has led to a reduction in the side increasingly become available in our environment, they effects of COCs including venous thrombosis and will lead to and increase in acceptance and use of myocardial infarction 6,7. -

Contraception Pearls for Practice
Contraception Pearls for Practice Academic Detailing Service Planning committee Content Experts Clinical reviewer Gillian Graves MD FRCS(C), Professor, Department of Obstetrics and Gynecology, Faculty of Medicine, Dalhousie University Drug evaluation pharmacist Pam McLean-Veysey BScPharm, Drug Evaluation Unit, Nova Scotia Health Family Physician Advisory Panel Bernie Buffett MD, Neils Harbour, Nova Scotia Ken Cameron BSc MD CCFP, Dartmouth, Nova Scotia Norah Mogan MD CCFP, Liverpool, Nova Scotia Dalhousie CPD Bronwen Jones MD CCFP – Family Physician, Director Evidence-based Programs in CPD, Associate Professor, Faculty of Medicine, Dalhousie University Michael Allen MD MSc – Family Physician, Professor, Post-retirement Appointment, Consultant Michael Fleming MD CCFP FCFP – Family Physician, Director Family Physician Programs in CPD Academic Detailers Isobel Fleming BScPharm ACPR, Director of Academic Detailing Service Lillian Berry BScPharm Julia Green-Clements BScPharm Kelley LeBlanc BScPharm Gabrielle Richard-McGibney BScPharm, BCPS, PharmD Cathy Ross RN BScNursing Thanks to Katie McLean, Librarian Educator, NSHA Central Zone for her help with literature searching. Cover artwork generated with Tagxedo.com Disclosure statements The Academic Detailing Service is operated by Dalhousie Continuing Professional Development, Faculty of Medicine and funded by the Nova Scotia Department of Health and Wellness. Dalhousie University Office of Continuing Professional Development has full control over content. Dr Bronwen Jones receives funding for her Academic Detailing work from the Nova Scotia Department of Health and Wellness. Dr Michael Allen has received funding from the Nova Scotia Department of Health and Wellness for research projects and to develop CME programs. Dr Gillian Graves has received funding for presentations from Actavis (Fibristal®) and is on the board of AbbVie (for Lupron®). -

NAMS Practice Pearl
NAMS Practice Pearl Extended Duration Use of Menopausal Hormone Therapy Released October 1, 2013 Andrew M. Kaunitz, MD, NCMP (University of Florida College of Medicine-Jacksonville, Department of Obstetrics & Gynecology, Jacksonville, FL) Although providing guidance to patients regarding duration of hormone therapy represents a topic surrounded by controversy, clinicians often encounter this issue in practice. As pointed out in the NAMS 2012 Hormone Therapy (HT) Position Statement, determining the optimal duration of HT is challenging both for clinicians and for patients. This Practice Pearl addresses clinical situations for which long-term HT might be appropriate and provides practical guidance regarding prudent therapeutic choices for women using HT for an extended duration. Use of Systemic HT to Treat Vasomotor Symptoms (VMS). Moderate to severe VMS represent the most common indication for systemic combination estrogen-progestin (EPT) or estrogen-only (ET) HT, and HT represents the most effective treatment for VMS.1 Some experts’ recommendations regarding HT duration of use have cautioned that “…it remains prudent to keep the… duration of treatment short” or that HT “…may serve a useful role in short-term symptom management.”2,3 However, VMS persist for longer than many have assumed. For instance, The Penn Ovarian Aging Study was conducted specifically to estimate the duration of moderate-to-severe VMS and found that median duration of such symptoms was 10.2 years. In this landmark cohort study, the median duration of VMS that started -

Contraception and Misconceptions
CONTRACEPTION AND MISCONCEPTIONS CONTRACEPTION IN WOMEN WITH MENTAL ILLNESS OVERVIEW Hormones and mood How mental illness impacts on contraceptive choice Ideal contraception Pro and cons of contraceptive methods in women with mental illness Hormonal contraception and mood ESTROGENS anti-inflammatory neuroprotective effects of estradiol modulation of the limbic processing memory of emotionally-relevant information. estradiol “beneficially” modulates pathways implicated in the pathophysiology of depression, including serotonin and norepinephrine pathways PROGESTROGENS Previously thought to be anxiogenic, depressogenic Breakdown products Depression – alpha hydroxy progestrogen breakdown products pro – inflammatory, anxiogenic HORMONES AND MOODS – COMPLEX INTERPLAY MENTAL ILLNESS AND CONTRACEPTIVE CHOICE Effect of mental illness on contraceptive choice Effect of contraceptive choice on mental illness Drug interactions EFFECT MENTAL ILLNESS ON CONTRACEPTIVE CHOICE Impulsive Poor planning Cognitive and problem judgement Poor adherence IMPULSIVITY COGNITION POOR JUDGEMENT LETS NOT FORGET SUBSTANCES…… TYPES OF CONTRACEPTION No method Non hormonal Condom/Femidom Diaphragm Non hormone containing IUCD (Copper T) Hormonal COC POP Mirena Evra Patch Nuva Ring Implant ORAL CONTRACEPTIVES POP – avoid Same time every day COC Pros Effective Reduction PMS – monophasic estrogen dominant pill eg Femodene, Yaz, Nordette Cons Drug interactions Pill burden Daily dose EVRA PATCH Weekly patch Transdermal system: 150 mcg/day -

209627Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209627Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review Reviewers of Multi-Disciplinary Review and Evaluation SECTIONS OFFICE/ AUTHORED/ ACKNOWLEDGED/ DISCIPLINE REVIEWER DIVISION APPROVED Mark Seggel, Ph.D. OPQ/ONDP/DNDP2 Authored: Section 4.2 Digitally signed by Mark R. Seggel -S CMC Lead DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, cn=Mark R. Signature: Mark R. Seggel -S Seggel -S, 0.9.2342.19200300.100.1.1=1300071539 Date: 2018.08.08 16:29:15 -04'00' Frederic Moulin, DVM, PhD OND/ODE3/DBRUP Authored: Section 5 Pharmacology/ Digitally signed by Frederic Moulin -S Toxicology DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Reviewer Signature: Frederic Moulin -S 0.9.2342.19200300.100.1.1=2001708658, cn=Frederic Moulin -S Date: 2018.08.08 15:26:57 -04'00' Kimberly Hatfield, PhD OND/ODE3/DBRUP Approved: Section 5 Pharmacology/ Toxicology Digitally signed by Kimberly P. Hatfield -S DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Team Leader Signature: Kimberly P. Hatfield -S 0.9.2342.19200300.100.1.1=1300387215, cn=Kimberly P. Hatfield -S Date: 2018.08.08 14:56:10 -04'00' Li Li, Ph.D. OCP/DCP3 Authored: Sections 6 and 17.3 Clinical Pharmacology Dig ta ly signed by Li Li S DN c=US o=U S Government ou=HHS ou=FDA ou=People Reviewer cn=Li Li S Signature: Li Li -S 0 9 2342 19200300 100 1 1=20005 08577 Date 2018 08 08 15 39 23 04'00' Doanh Tran, Ph.D. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
Morbidity and Mortality Weekly Report Recommendations and Reports / Vol. 65 / No. 3 July 29, 2016 U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 U.S. Department of Health and Human Services Centers for Disease Control and Prevention Recommendations and Reports CONTENTS Introduction ............................................................................................................1 Methods ....................................................................................................................2 How to Use This Document ...............................................................................3 Keeping Guidance Up to Date ..........................................................................5 References ................................................................................................................8 Abbreviations and Acronyms ............................................................................9 Appendix A: Summary of Changes from U.S. Medical Eligibility Criteria for Contraceptive Use, 2010 ...........................................................................10 Appendix B: Classifications for Intrauterine Devices ............................. 18 Appendix C: Classifications for Progestin-Only Contraceptives ........ 35 Appendix D: Classifications for Combined Hormonal Contraceptives .... 55 Appendix E: Classifications for Barrier Methods ..................................... 81 Appendix F: Classifications for Fertility Awareness–Based Methods ..... 88 Appendix G: Lactational -

Transdermal Versus Oral Estrogen: Clinical Outcomes in Patients
Journal of Assisted Reproduction and Genetics https://doi.org/10.1007/s10815-018-1380-5 ASSISTED REPRODUCTION TECHNOLOGIES Transdermal versus oral estrogen: clinical outcomes in patients undergoing frozen-thawed single blastocyst transfer cycles without GnRHa suppression, a prospective randomized clinical trial Semra Kahraman1 & Caroline Pirkevi Çetinkaya1 & Yucel Sahin1 & Gokalp Oner1 Received: 20 September 2018 /Accepted: 26 November 2018 # The Author(s) 2018 Abstract Purpose To conduct a non-inferiority study to compare the clinical outcomes of transdermal estrogen patch and oral estrogen in patients undergoing frozen-thawed single blastocyst transfer non-donor cycles without GnRHagonist (GnRHa) suppression. Methods A total of 317 women with irregular menses or anovulatory cycle undergoing frozen-thawed embryo transfer (FET) non-donor cycles without GnRHa suppression were involved in a prospective randomized clinical trial between May 2017 and October 2017. The trial was conducted in an ART and Reproductive Genetics Centre within a private hospital. The unit is designated as a teaching center by the Turkish Ministry of Health. Oral or transdermal estrogen was administered in patients undergoing frozen-thawed single blastocyst transfer. The outcomes of the study were the following: endometrial thickness on the day of progesterone administration, implantation rate, and clinical and viable ongoing pregnancy rates. Results Endometrial thickness and clinical outcomes of oral and transdermal estrogen administration were equally successful (p >0.05). Conclusion No significant difference was found in endometrial thickness on the day of progesterone administration nor in clinical outcomes between transdermal estrogen and oral estrogen in patients undergoing frozen-thawed single blastocyst stage transfer cycles without GnRHa suppression. Keywords Oral estrogen . -

Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2Nd Edition
Morbidity and Mortality Weekly Report Early Release / Vol. 62 June 14, 2013 U.S. Selected Practice Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd Edition Continuing Education Examination available at http://www.cdc.gov/mmwr/cme/conted.html. U.S. Department of Health and Human Services Centers for Disease Control and Prevention Early Release CONTENTS CONTENTS (Continued) Introduction ............................................................................................................1 Appendix A: Summary Chart of U.S. Medical Eligibility Criteria for Methods ....................................................................................................................2 Contraceptive Use, 2010 .................................................................................. 47 How To Use This Document ...............................................................................3 Appendix B: When To Start Using Specific Contraceptive Summary of Changes from WHO SPR ............................................................4 Methods .............................................................................................................. 55 Contraceptive Method Choice .........................................................................4 Appendix C: Examinations and Tests Needed Before Initiation of Maintaining Updated Guidance ......................................................................4 Contraceptive Methods -
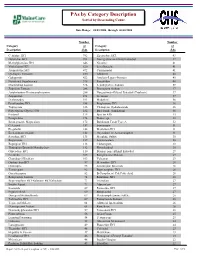
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees. -

The History of Estrogen Therapy
REVIEW The History of Estrogen Therapy Grace E. Kohn,1 Katherine M. Rodriguez, MD,2 James Hotaling, MD,3 and Alexander W. Pastuszak, MD, PhD3 ABSTRACT Introduction: Menopausal hormone therapy (MHT) has proven an effective treatment for the amelioration of symptoms of menopause. The idea that a substance was the missing factor in a woman’s body after menopause dates to the 1800s, when cow ovarian tissue was injected into German women in a successful attempt to reverse the sexual symptoms of menopause. The early 1900s saw the rise of commercialized menopause “treatments” that ranged in substance and even theoretical efficacy. The role of estrogen was first accurately described in Guinea pigs in 1917 by Dr. Papanicolaou. Aim: To tell the detailed history of how estrogen was discovered and the controversy surrounding MHT. Methods: A literature search was conducted using PubMed to identify relevant studies and historical documents regarding the history of estrogen therapy. Results: The history of estrogen supplementation and its controversies are interesting stories and relevant to today’s ongoing investigation into hormone replacement. Conclusion: The controversy of MHT remained until the first randomized trials examining MHT in the early 1990s that suggested MHT is cardioprotective in postmenopausal women, although this conclusion was contradicted in subsequent trials. In the present day, MHT is approved only for short-term use for the symptomatic treatment of menopause. Kohn GE, Rodriguez KM, Hotaling J, et al. The History of Estrogen Therapy. Sex Med Rev 2019;XX:XXXeXXX. Copyright Ó 2019, International Society for Sexual Medicine. Published by Elsevier Inc. All rights reserved.