The Effect of Dietary Mushroom Agaricus Bisporus on Intestinal Microbiota Composition and Host Immunological Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Nutrient Composition of Selected Wild Edible Mushrooms from Two Agro‑Ecological Zones, Uganda
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Nakalembe et al. SpringerPlus (2015) 4:433 DOI 10.1186/s40064-015-1188-z RESEARCH Open Access Comparative nutrient composition of selected wild edible mushrooms from two agro‑ecological zones, Uganda Immaculate Nakalembe1*, John David Kabasa2 and Deogratias Olila3 *Correspondence: immynakalembe@covab. Abstract mak.ac.ug In Uganda, wild mushrooms are mainly collected during the rainy season and valued 1 Department of Biomolecular Resources as a traditionally nutritious food by the rural poor. However, their nutritional attributes and Biolaboratory Sciences, have not been adequately studied and documented. Comparative nutrient composi- Makerere University, P. O. tion of five wild edible mushroom species was determined, namely: P. tenucuilus, T. Box 7062, Kampala, Uganda Full list of author information tyleranus, T. clypeatus, V. speciosa and T. microcarpus of sub-humid and humid agro- is available at the end of the ecological zones. Standard analytical techniques following the AOAC were used for article proximate and mineral contents determinations. Vitamins determination followed the established standard protocols of the laboratories where the analyses were conducted. Combined use of nutrient concentration and scores were used to compare the level of the contents in the mushroom species. Significant differences (p < 0.05) in nutrient values were demonstrated between and among the mushroom species obtained from the two agro-ecological zones. On dry weight basis, all proximate compositions were high in mushroom species obtained from the humid zone with exception of the total carbohydrates and energy values. Irrespective of the source of the mushrooms, signifi- cant amounts were demonstrated in protein, dry matter, ash and total carbohydrates ranging between 11.56–27.42%, 82.34–99.76%, 10.79–16.87%, and 37.12–61.05%, respectively. -

Xiexin Tang Improves the Symptom of Type 2 Diabetic Rats by Modulation of the Gut Microbiota
www.nature.com/scientificreports OPEN Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota Received: 30 August 2017 Xiaoyan Wei, Jinhua Tao , Suwei Xiao, Shu Jiang, Erxin Shang, Zhenhua Zhu, Dawei Qian Accepted: 13 February 2018 & Jinao Duan Published: xx xx xxxx Type 2 diabetes mellitus (T2DM), a chronic metabolic disease which severely impairs peoples’ quality of life, currently attracted worldwide concerns. There are growing evidences that gut microbiota can exert a great impact on the development of T2DM. Xiexin Tang (XXT), a traditional Chinese medicine prescription, has been clinically used to treat diabetes for thousands of years. However, few researches are investigated on the modulation of gut microbiota community by XXT which will be very helpful to unravel how it works. In this study, bacterial communities were analyzed based on high-throughput 16S rRNA gene sequencing. Results indicated that XXT could notably shape the gut microbiota. T2DM rats treated with XXT exhibited obvious changes in the composition of the gut microbiota, especially for some short chain fatty acids producing and anti-infammatory bacteria such as Adlercreutzia, Alloprevotella, Barnesiella, [Eubacterium] Ventriosum group, Blautia, Lachnospiraceae UCG-001, Papillibacter and Prevotellaceae NK3B31 group. Additionally, XXT could also signifcantly ameliorate hyperglycemia, lipid metabolism dysfunction and infammation in T2DM rats. Moreover, the correlation analysis illustrated that the key microbiota had a close relationship with the T2DM related indexes. The results probably provided useful information for further investigation on its active mechanism and clinical application. T2DM, a chronic metabolic disease characterized by hyperglycemia as a result of insufcient insulin secretion, insulin action or both1, is estimated that its numbers in the adults will increase by 55% by 20352. -

Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease
biomedicines Article Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease Ashani Lecamwasam 1,2,3,*, Tiffanie M. Nelson 4, Leni Rivera 3, Elif I. Ekinci 2,5, Richard Saffery 1,6 and Karen M. Dwyer 3 1 Epigenetics Research, Murdoch Children’s Research Institute, VIC 3052, Australia; [email protected] 2 Department of Endocrinology, Austin Health, VIC 3079, Australia; [email protected] 3 School of Medicine, Faculty of Health, Deakin University, VIC 3220, Australia; [email protected] (L.R.); [email protected] (K.M.D.) 4 Menzies Health Institute Queensland, Griffith University, QLD 4222, Australia; [email protected] 5 Department of Medicine, University of Melbourne, VIC 3010, Australia 6 Department of Paediatrics, University of Melbourne, VIC 3010, Australia * Correspondence: [email protected]; Tel.: +613-8341-6200; Fax: +613-9348-1391 Abstract: (1) Background: Individuals with diabetes and chronic kidney disease display gut dysbiosis when compared to healthy controls. However, it is unknown whether there is a change in dysbiosis across the stages of diabetic chronic kidney disease. We investigated a cross-sectional study of patients with early and late diabetes associated chronic kidney disease to identify possible microbial differences between these two groups and across each of the stages of diabetic chronic kidney disease. (2) Methods: This cross-sectional study recruited 95 adults. DNA extracted from collected stool samples were used for 16S rRNA sequencing to identify the bacterial community in the Citation: Lecamwasam, A.; Nelson, gut. (3) Results: The phylum Firmicutes was the most abundant and its mean relative abundance T.M.; Rivera, L.; Ekinci, E.I.; Saffery, was similar in the early and late chronic kidney disease group, 45.99 ± 0.58% and 49.39 ± 0.55%, R.; Dwyer, K.M. -
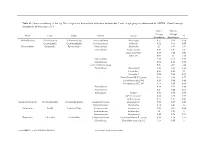
Genus Contributing to the Top 70% of Significant Dissimilarity of Bacteria Between Day 7 and 18 Age Groups As Determined by SIMPER
Table S1: Genus contributing to the top 70% of significant dissimilarity of bacteria between day 7 and 18 age groups as determined by SIMPER. Overall average dissimilarity between ages is 51%. Day 7 Day 18 Average Average Phyla Class Order Family Genus % Abundance Abundance Actinobacteria Actinobacteria Actinomycetales Actinomycetaceae Actinomyces 0.57 0.24 0.74 Coriobacteriia Coriobacteriales Coriobacteriaceae Collinsella 0.51 0.61 0.57 Bacteroidetes Bacteroidia Bacteroidales Bacteroidaceae Bacteroides 2.1 1.63 1.03 Marinifilaceae Butyricimonas 0.82 0.81 0.9 Sanguibacteroides 0.08 0.42 0.59 CAG-873 0.01 1.1 1.68 Marinifilaceae 0.38 0.78 0.91 Marinifilaceae 0.78 1.04 0.97 p-2534-18B5 gut group 0.06 0.7 1.04 Prevotellaceae Alloprevotella 0.46 0.83 0.89 Prevotella 2 0.95 1.11 1.3 Prevotella 7 0.22 0.42 0.56 Prevotellaceae NK3B31 group 0.62 0.68 0.77 Prevotellaceae UCG-003 0.26 0.64 0.89 Prevotellaceae UCG-004 0.11 0.48 0.68 Prevotellaceae 0.64 0.52 0.89 Prevotellaceae 0.27 0.42 0.55 Rikenellaceae Alistipes 0.39 0.62 0.77 dgA-11 gut group 0.04 0.38 0.56 RC9 gut group 0.79 1.17 0.95 Epsilonbacteraeota Campylobacteria Campylobacterales Campylobacteraceae Campylobacter 0.58 0.83 0.97 Helicobacteraceae Helicobacter 0.12 0.41 0.6 Firmicutes Bacilli Lactobacillales Enterococcaceae Enterococcus 0.36 0.31 0.64 Lactobacillaceae Lactobacillus 1.4 1.24 1 Streptococcaceae Streptococcus 0.82 0.58 0.53 Firmicutes Clostridia Clostridiales Christensenellaceae Christensenellaceae R-7 group 0.38 1.36 1.56 Clostridiaceae Clostridium sensu stricto 1 1.16 0.58 -

First Cultivation of Agaricus Flocculosipes and a Novel Thai Strain of A
Mycosphere 5 (6): 814–820 (2014) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2014 Online Edition Doi 10.5943/mycosphere/5/6/11 First cultivation of Agaricus flocculosipes and a novel Thai strain of A. subrufescens Thongklang N 1, 2, Sysouphanthong P 3, Callac P 4 and Hyde KD 1,2 1School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 2Institute of Excellence in Fungal Research, and School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 3Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, China 4UR 1264, Mycologie et Sécurité des Aliments, 33883 Villenave d’ Ornon, France Thongklang N, Sysouphanthong P, Callac P, Hyde KD 2014 – First cultivation of Agaricus flocculosipes and a novel Thai strain of A. subrufescens. Mycosphere 5(6), 814–820, Doi 10.5943/mycosphere/5/6/11 Abstract Agaricus flocculosipes and A. subrufescens are edible species that belong to section Arvenses of the genus Agaricus. Agaricus subrufescens (almond mushroom) is known to produce bioactive compounds with medicinal properties, such as anti-cancer and anti-tumor activity and fruiting bodies are also edible and nutritious. Agaricus subrufescens is presently cultivated in Brazil, China, Japan, Taiwan and some European countries for use as foods and nutraceuticals. Agaricus flocculosipes is a newly described species currently known only from Thailand, Mayotte Island and China. Species of Agaricus have high potential for cultivation as many species are edible and have medicinal properties. Herein we report the first cultivation of A. flocculosipes and a Thai strain of A. -

Role of Actinobacteria and Coriobacteriia in the Antidepressant Effects of Ketamine in an Inflammation Model of Depression
Pharmacology, Biochemistry and Behavior 176 (2019) 93–100 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression T Niannian Huanga,1, Dongyu Huaa,1, Gaofeng Zhana, Shan Lia, Bin Zhub, Riyue Jiangb, Ling Yangb, ⁎ ⁎ Jiangjiang Bia, Hui Xua, Kenji Hashimotoc, Ailin Luoa, , Chun Yanga, a Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China b Department of Internal Medicine, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China c Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba 260-8670, Japan ARTICLE INFO ABSTRACT Keywords: Ketamine, an N-methyl-D-aspartic acid receptor (NMDAR) antagonist, elicits rapid-acting and sustained anti- Ketamine depressant effects in treatment-resistant depressed patients. Accumulating evidence suggests that gut microbiota Depression via the gut-brain axis play a role in the pathogenesis of depression, thereby contributing to the antidepressant Lipopolysaccharide actions of certain compounds. Here we investigated the role of gut microbiota in the antidepressant effects of Gut microbiota ketamine in lipopolysaccharide (LPS)-induced inflammation model of depression. Ketamine (10 mg/kg) sig- nificantly attenuated the increased immobility time in forced swimming test (FST), which was associated with the improvements in α-diversity, consisting of Shannon, Simpson and Chao 1 indices. In addition to α-diversity, β-diversity, such as principal coordinates analysis (PCoA), and linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe), showed a differential profile after ketamine treatment. -

Gut Microbiota Differs in Composition and Functionality Between Children
Diabetes Care Volume 41, November 2018 2385 Gut Microbiota Differs in Isabel Leiva-Gea,1 Lidia Sanchez-Alcoholado,´ 2 Composition and Functionality Beatriz Mart´ın-Tejedor,1 Daniel Castellano-Castillo,2,3 Between Children With Type 1 Isabel Moreno-Indias,2,3 Antonio Urda-Cardona,1 Diabetes and MODY2 and Healthy Francisco J. Tinahones,2,3 Jose´ Carlos Fernandez-Garc´ ´ıa,2,3 and Control Subjects: A Case-Control Mar´ıa Isabel Queipo-Ortuno~ 2,3 Study Diabetes Care 2018;41:2385–2395 | https://doi.org/10.2337/dc18-0253 OBJECTIVE Type 1 diabetes is associated with compositional differences in gut microbiota. To date, no microbiome studies have been performed in maturity-onset diabetes of the young 2 (MODY2), a monogenic cause of diabetes. Gut microbiota of type 1 diabetes, MODY2, and healthy control subjects was compared. PATHOPHYSIOLOGY/COMPLICATIONS RESEARCH DESIGN AND METHODS This was a case-control study in 15 children with type 1 diabetes, 15 children with MODY2, and 13 healthy children. Metabolic control and potential factors mod- ifying gut microbiota were controlled. Microbiome composition was determined by 16S rRNA pyrosequencing. 1Pediatric Endocrinology, Hospital Materno- Infantil, Malaga,´ Spain RESULTS 2Clinical Management Unit of Endocrinology and Compared with healthy control subjects, type 1 diabetes was associated with a Nutrition, Laboratory of the Biomedical Research significantly lower microbiota diversity, a significantly higher relative abundance of Institute of Malaga,´ Virgen de la Victoria Uni- Bacteroides Ruminococcus Veillonella Blautia Streptococcus versityHospital,Universidad de Malaga,M´ alaga,´ , , , , and genera, and a Spain lower relative abundance of Bifidobacterium, Roseburia, Faecalibacterium, and 3Centro de Investigacion´ BiomedicaenRed(CIBER)´ Lachnospira. -

Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans
microorganisms Article Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans Sandi L. Navarro 1,* , Lisa Levy 1, Keith R. Curtis 1, Johanna W. Lampe 1,2 and Meredith A.J. Hullar 1 1 Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] (L.L.); [email protected] (K.R.C.); [email protected] (J.W.L.); [email protected] (M.A.J.H.) 2 Department of Epidemiology, University of Washington, Seattle, WA 98195, USA * Correspondence: [email protected]; Tel.: +1-206-667-6583 Received: 31 October 2019; Accepted: 22 November 2019; Published: 23 November 2019 Abstract: Glucosamine and chondroitin (G&C), typically taken for joint pain, are among the most frequently used specialty supplements by US adults. More recently, G&C have been associated with lower incidence of colorectal cancer in human observational studies and reduced severity of experimentally-induced ulcerative colitis in rodents. However, little is known about their effects on colon-related physiology. G&C are poorly absorbed and therefore metabolized by gut microbiota. G&C have been associated with changes in microbial structure, which may alter host response. We conducted a randomized, double-blind, placebo-controlled crossover trial in ten healthy adults to evaluate the effects of a common dose of G&C compared to placebo for 14 days on gut microbial community structure, measured by 16S rRNA gene sequencing. Linear mixed models were used to evaluate the effect of G&C compared to placebo on fecal microbial alpha and beta diversity, seven phyla, and 137 genera. -

Small Scale Mushroom Production Agaricus Bisporus
Small Scale Mushroom Production Agaricus bisporus VEGETABLE CROPS PRODUCTION GUIDE FOR THE ATLANTIC PROVINCES Prepared by the ADVISORY COMMITTEE ON VEGETABLE CROPS Published by authority of the ATLANTIC PROVINCES AGRICULTURE SERVICES CO-ORDINATING COMMITTEE Introduction Successful mushroom growing involves overcoming difficulties such as temperature and humidity control, pest control and compost preparation. The amateur mushroom grower should recognize that most basements do not provide ideal conditions for good growth. Mushroom production is a difficult task at the best of times. This publication is intended to provide useful tips in order to increase the rate of success of growing mushrooms. Location For the amateur, mushrooms are usually planted in the fall and the best location is the cellar, basement or a barn or any tight, light-proof, well ventilated and insulated building. The following conditions should be met: 1.Air temperatures controlled between 13/C and 21/C. 2.Relative humidities between 80-95 %. A corner of the basement can be partitioned off by the use of a polyethylene divider. This will help to maintain proper humidity levels. A plastic hood placed over the growing bed is a second alternative. Do not place beds where direct sunlight will fall on them. Ventilation is useful to remove offensive odors. Where temperatures cannot be maintained, supplementary heat is necessary. Mushroom beds are usually 120-150 cm wide, 15-20 cm deep and as long as you wish. Boards that form the bottom should not be over 15-20 cm wide, leaving 2 cm to 4 cm cracks between them for ventilation. Several tiers can be made approximately 60 cm apart. -

Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella Bryantii B14
proteomes Article Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella bryantii B14 Andrej Trautmann 1, Lena Schleicher 2, Simon Deusch 1 , Jochem Gätgens 3 , Julia Steuber 2 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany; [email protected] (A.T.); [email protected] (S.D.) 2 Institute of Biology, University of Hohenheim, 70599 Stuttgart, Germany; [email protected] (L.S.); [email protected] (J.S.) 3 Institute of Bio- and Geosciences, IBG-1: Biotechnology, Forschungszentrum Jülich, 52425 Jülich, Germany; [email protected] * Correspondence: [email protected] Received: 22 September 2020; Accepted: 13 October 2020; Published: 16 October 2020 Abstract: Short-chain fatty acids (SCFAs) are bacterial products that are known to be used as energy sources in eukaryotic hosts, whereas their role in the metabolism of intestinal microbes is rarely explored. In the present study, acetic, propionic, butyric, isobutyric, valeric, and isovaleric acid, respectively, were added to a newly defined medium containing Prevotella bryantii B14 cells. After 8 h and 24 h, optical density, pH and SCFA concentrations were measured. Long-chain fatty acid (LCFA) profiles of the bacterial cells were analyzed via gas chromatography-time of flight-mass spectrometry (GC-ToF MS) and proteins were quantified using a mass spectrometry-based, label-free approach. Cultures supplemented with single SCFAs revealed different growth behavior. Structural features of the respective SCFAs were identified in the LCFA profiles, which suggests incorporation into the bacterial membranes. The proteomes of cultures supplemented with acetic and valeric acid differed by an increased abundance of outer membrane proteins. -

Prevotella Multisaccharivorax Type Strain (PPPA20T)
Lawrence Berkeley National Laboratory Recent Work Title Non-contiguous finished genome sequence of the opportunistic oral pathogen Prevotella multisaccharivorax type strain (PPPA20). Permalink https://escholarship.org/uc/item/0p79h5ds Journal Standards in genomic sciences, 5(1) ISSN 1944-3277 Authors Pati, Amrita Gronow, Sabine Lu, Megan et al. Publication Date 2011-10-01 DOI 10.4056/sigs.2164949 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Standards in Genomic Sciences (2011) 5:41-49 DOI:10.4056/sigs.2164949 Non-contiguous finished genome sequence of the opportunistic oral pathogen Prevotella multisaccharivorax type strain (PPPA20T) Amrita Pati1, Sabine Gronow2, Megan Lu1,3, Alla Lapidus1, Matt Nolan1, Susan Lucas1, Nancy Hammon1, Shweta Deshpande1, Jan-Fang Cheng1, Roxanne Tapia1,3, Cliff Han1,3, Lynne Goodwin1,3 Sam Pitluck1, Konstantinos Liolios1, Ioanna Pagani1, Konstantinos Mavromatis1, Natalia Mikhailova1, Marcel Huntemann1, Amy Chen4, Krishna Palaniappan4, Miriam Land1,5, Loren Hauser1,5, John C. Detter1,3, Evelyne-Marie Brambilla2, Manfred Rohde6, Markus Göker2, Tanja Woyke1, James Bristow1, Jonathan A. Eisen1,7, Victor Markowitz4, Philip Hugenholtz1,8, Nikos C. Kyrpides1, Hans-Peter Klenk2*, and Natalia Ivanova1 1 DOE Joint Genome Institute, Walnut Creek, California, USA 2 DSMZ - German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany 3 Los Alamos National Laboratory, Bioscience Division, Los Alamos, New Mexico, USA 4 Biological Data Management and -

Characterization of Antibiotic Resistance Genes in the Species of the Rumen Microbiota
ARTICLE https://doi.org/10.1038/s41467-019-13118-0 OPEN Characterization of antibiotic resistance genes in the species of the rumen microbiota Yasmin Neves Vieira Sabino1, Mateus Ferreira Santana1, Linda Boniface Oyama2, Fernanda Godoy Santos2, Ana Júlia Silva Moreira1, Sharon Ann Huws2* & Hilário Cuquetto Mantovani 1* Infections caused by multidrug resistant bacteria represent a therapeutic challenge both in clinical settings and in livestock production, but the prevalence of antibiotic resistance genes 1234567890():,; among the species of bacteria that colonize the gastrointestinal tract of ruminants is not well characterized. Here, we investigate the resistome of 435 ruminal microbial genomes in silico and confirm representative phenotypes in vitro. We find a high abundance of genes encoding tetracycline resistance and evidence that the tet(W) gene is under positive selective pres- sure. Our findings reveal that tet(W) is located in a novel integrative and conjugative element in several ruminal bacterial genomes. Analyses of rumen microbial metatranscriptomes confirm the expression of the most abundant antibiotic resistance genes. Our data provide insight into antibiotic resistange gene profiles of the main species of ruminal bacteria and reveal the potential role of mobile genetic elements in shaping the resistome of the rumen microbiome, with implications for human and animal health. 1 Departamento de Microbiologia, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil. 2 Institute for Global Food Security, School of Biological