Xiexin Tang Improves the Symptom of Type 2 Diabetic Rats by Modulation of the Gut Microbiota
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2018/064165 A2 (.Pdf)
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/064165 A2 05 April 2018 (05.04.2018) W !P O PCT (51) International Patent Classification: Published: A61K 35/74 (20 15.0 1) C12N 1/21 (2006 .01) — without international search report and to be republished (21) International Application Number: upon receipt of that report (Rule 48.2(g)) PCT/US2017/053717 — with sequence listing part of description (Rule 5.2(a)) (22) International Filing Date: 27 September 2017 (27.09.2017) (25) Filing Language: English (26) Publication Langi English (30) Priority Data: 62/400,372 27 September 2016 (27.09.2016) US 62/508,885 19 May 2017 (19.05.2017) US 62/557,566 12 September 2017 (12.09.2017) US (71) Applicant: BOARD OF REGENTS, THE UNIVERSI¬ TY OF TEXAS SYSTEM [US/US]; 210 West 7th St., Austin, TX 78701 (US). (72) Inventors: WARGO, Jennifer; 1814 Bissonnet St., Hous ton, TX 77005 (US). GOPALAKRISHNAN, Vanch- eswaran; 7900 Cambridge, Apt. 10-lb, Houston, TX 77054 (US). (74) Agent: BYRD, Marshall, P.; Parker Highlander PLLC, 1120 S. Capital Of Texas Highway, Bldg. One, Suite 200, Austin, TX 78746 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease
biomedicines Article Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease Ashani Lecamwasam 1,2,3,*, Tiffanie M. Nelson 4, Leni Rivera 3, Elif I. Ekinci 2,5, Richard Saffery 1,6 and Karen M. Dwyer 3 1 Epigenetics Research, Murdoch Children’s Research Institute, VIC 3052, Australia; [email protected] 2 Department of Endocrinology, Austin Health, VIC 3079, Australia; [email protected] 3 School of Medicine, Faculty of Health, Deakin University, VIC 3220, Australia; [email protected] (L.R.); [email protected] (K.M.D.) 4 Menzies Health Institute Queensland, Griffith University, QLD 4222, Australia; [email protected] 5 Department of Medicine, University of Melbourne, VIC 3010, Australia 6 Department of Paediatrics, University of Melbourne, VIC 3010, Australia * Correspondence: [email protected]; Tel.: +613-8341-6200; Fax: +613-9348-1391 Abstract: (1) Background: Individuals with diabetes and chronic kidney disease display gut dysbiosis when compared to healthy controls. However, it is unknown whether there is a change in dysbiosis across the stages of diabetic chronic kidney disease. We investigated a cross-sectional study of patients with early and late diabetes associated chronic kidney disease to identify possible microbial differences between these two groups and across each of the stages of diabetic chronic kidney disease. (2) Methods: This cross-sectional study recruited 95 adults. DNA extracted from collected stool samples were used for 16S rRNA sequencing to identify the bacterial community in the Citation: Lecamwasam, A.; Nelson, gut. (3) Results: The phylum Firmicutes was the most abundant and its mean relative abundance T.M.; Rivera, L.; Ekinci, E.I.; Saffery, was similar in the early and late chronic kidney disease group, 45.99 ± 0.58% and 49.39 ± 0.55%, R.; Dwyer, K.M. -
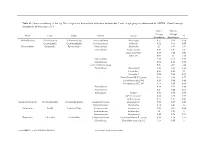
Genus Contributing to the Top 70% of Significant Dissimilarity of Bacteria Between Day 7 and 18 Age Groups As Determined by SIMPER
Table S1: Genus contributing to the top 70% of significant dissimilarity of bacteria between day 7 and 18 age groups as determined by SIMPER. Overall average dissimilarity between ages is 51%. Day 7 Day 18 Average Average Phyla Class Order Family Genus % Abundance Abundance Actinobacteria Actinobacteria Actinomycetales Actinomycetaceae Actinomyces 0.57 0.24 0.74 Coriobacteriia Coriobacteriales Coriobacteriaceae Collinsella 0.51 0.61 0.57 Bacteroidetes Bacteroidia Bacteroidales Bacteroidaceae Bacteroides 2.1 1.63 1.03 Marinifilaceae Butyricimonas 0.82 0.81 0.9 Sanguibacteroides 0.08 0.42 0.59 CAG-873 0.01 1.1 1.68 Marinifilaceae 0.38 0.78 0.91 Marinifilaceae 0.78 1.04 0.97 p-2534-18B5 gut group 0.06 0.7 1.04 Prevotellaceae Alloprevotella 0.46 0.83 0.89 Prevotella 2 0.95 1.11 1.3 Prevotella 7 0.22 0.42 0.56 Prevotellaceae NK3B31 group 0.62 0.68 0.77 Prevotellaceae UCG-003 0.26 0.64 0.89 Prevotellaceae UCG-004 0.11 0.48 0.68 Prevotellaceae 0.64 0.52 0.89 Prevotellaceae 0.27 0.42 0.55 Rikenellaceae Alistipes 0.39 0.62 0.77 dgA-11 gut group 0.04 0.38 0.56 RC9 gut group 0.79 1.17 0.95 Epsilonbacteraeota Campylobacteria Campylobacterales Campylobacteraceae Campylobacter 0.58 0.83 0.97 Helicobacteraceae Helicobacter 0.12 0.41 0.6 Firmicutes Bacilli Lactobacillales Enterococcaceae Enterococcus 0.36 0.31 0.64 Lactobacillaceae Lactobacillus 1.4 1.24 1 Streptococcaceae Streptococcus 0.82 0.58 0.53 Firmicutes Clostridia Clostridiales Christensenellaceae Christensenellaceae R-7 group 0.38 1.36 1.56 Clostridiaceae Clostridium sensu stricto 1 1.16 0.58 -

Longitudinal Characterization of the Gut Bacterial and Fungal Communities in Yaks
Journal of Fungi Article Longitudinal Characterization of the Gut Bacterial and Fungal Communities in Yaks Yaping Wang 1,2,3, Yuhang Fu 3, Yuanyuan He 3, Muhammad Fakhar-e-Alam Kulyar 3 , Mudassar Iqbal 3,4, Kun Li 1,2,* and Jiaguo Liu 1,2,* 1 Institute of Traditional Chinese Veterinary Medicine, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China; [email protected] 2 MOE Joint International Research Laboratory of Animal Health and Food Safety, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China 3 College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, China; [email protected] (Y.F.); [email protected] (Y.H.); [email protected] (M.F.-e.-A.K.); [email protected] (M.I.) 4 Faculty of Veterinary and Animal Sciences, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan * Correspondence: [email protected] (K.L.); [email protected] (J.L.) Abstract: Development phases are important in maturing immune systems, intestinal functions, and metabolism for the construction, structure, and diversity of microbiome in the intestine during the entire life. Characterizing the gut microbiota colonization and succession based on age-dependent effects might be crucial if a microbiota-based therapeutic or disease prevention strategy is adopted. The purpose of this study was to reveal the dynamic distribution of intestinal bacterial and fungal communities across all development stages in yaks. Dynamic changes (a substantial difference) in the structure and composition ratio of the microbial community were observed in yaks that Citation: Wang, Y.; Fu, Y.; He, Y.; matched the natural aging process from juvenile to natural aging. -

Role of Actinobacteria and Coriobacteriia in the Antidepressant Effects of Ketamine in an Inflammation Model of Depression
Pharmacology, Biochemistry and Behavior 176 (2019) 93–100 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression T Niannian Huanga,1, Dongyu Huaa,1, Gaofeng Zhana, Shan Lia, Bin Zhub, Riyue Jiangb, Ling Yangb, ⁎ ⁎ Jiangjiang Bia, Hui Xua, Kenji Hashimotoc, Ailin Luoa, , Chun Yanga, a Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China b Department of Internal Medicine, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China c Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba 260-8670, Japan ARTICLE INFO ABSTRACT Keywords: Ketamine, an N-methyl-D-aspartic acid receptor (NMDAR) antagonist, elicits rapid-acting and sustained anti- Ketamine depressant effects in treatment-resistant depressed patients. Accumulating evidence suggests that gut microbiota Depression via the gut-brain axis play a role in the pathogenesis of depression, thereby contributing to the antidepressant Lipopolysaccharide actions of certain compounds. Here we investigated the role of gut microbiota in the antidepressant effects of Gut microbiota ketamine in lipopolysaccharide (LPS)-induced inflammation model of depression. Ketamine (10 mg/kg) sig- nificantly attenuated the increased immobility time in forced swimming test (FST), which was associated with the improvements in α-diversity, consisting of Shannon, Simpson and Chao 1 indices. In addition to α-diversity, β-diversity, such as principal coordinates analysis (PCoA), and linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe), showed a differential profile after ketamine treatment. -

Gut Microbiota Differs in Composition and Functionality Between Children
Diabetes Care Volume 41, November 2018 2385 Gut Microbiota Differs in Isabel Leiva-Gea,1 Lidia Sanchez-Alcoholado,´ 2 Composition and Functionality Beatriz Mart´ın-Tejedor,1 Daniel Castellano-Castillo,2,3 Between Children With Type 1 Isabel Moreno-Indias,2,3 Antonio Urda-Cardona,1 Diabetes and MODY2 and Healthy Francisco J. Tinahones,2,3 Jose´ Carlos Fernandez-Garc´ ´ıa,2,3 and Control Subjects: A Case-Control Mar´ıa Isabel Queipo-Ortuno~ 2,3 Study Diabetes Care 2018;41:2385–2395 | https://doi.org/10.2337/dc18-0253 OBJECTIVE Type 1 diabetes is associated with compositional differences in gut microbiota. To date, no microbiome studies have been performed in maturity-onset diabetes of the young 2 (MODY2), a monogenic cause of diabetes. Gut microbiota of type 1 diabetes, MODY2, and healthy control subjects was compared. PATHOPHYSIOLOGY/COMPLICATIONS RESEARCH DESIGN AND METHODS This was a case-control study in 15 children with type 1 diabetes, 15 children with MODY2, and 13 healthy children. Metabolic control and potential factors mod- ifying gut microbiota were controlled. Microbiome composition was determined by 16S rRNA pyrosequencing. 1Pediatric Endocrinology, Hospital Materno- Infantil, Malaga,´ Spain RESULTS 2Clinical Management Unit of Endocrinology and Compared with healthy control subjects, type 1 diabetes was associated with a Nutrition, Laboratory of the Biomedical Research significantly lower microbiota diversity, a significantly higher relative abundance of Institute of Malaga,´ Virgen de la Victoria Uni- Bacteroides Ruminococcus Veillonella Blautia Streptococcus versityHospital,Universidad de Malaga,M´ alaga,´ , , , , and genera, and a Spain lower relative abundance of Bifidobacterium, Roseburia, Faecalibacterium, and 3Centro de Investigacion´ BiomedicaenRed(CIBER)´ Lachnospira. -

Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans
microorganisms Article Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans Sandi L. Navarro 1,* , Lisa Levy 1, Keith R. Curtis 1, Johanna W. Lampe 1,2 and Meredith A.J. Hullar 1 1 Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] (L.L.); [email protected] (K.R.C.); [email protected] (J.W.L.); [email protected] (M.A.J.H.) 2 Department of Epidemiology, University of Washington, Seattle, WA 98195, USA * Correspondence: [email protected]; Tel.: +1-206-667-6583 Received: 31 October 2019; Accepted: 22 November 2019; Published: 23 November 2019 Abstract: Glucosamine and chondroitin (G&C), typically taken for joint pain, are among the most frequently used specialty supplements by US adults. More recently, G&C have been associated with lower incidence of colorectal cancer in human observational studies and reduced severity of experimentally-induced ulcerative colitis in rodents. However, little is known about their effects on colon-related physiology. G&C are poorly absorbed and therefore metabolized by gut microbiota. G&C have been associated with changes in microbial structure, which may alter host response. We conducted a randomized, double-blind, placebo-controlled crossover trial in ten healthy adults to evaluate the effects of a common dose of G&C compared to placebo for 14 days on gut microbial community structure, measured by 16S rRNA gene sequencing. Linear mixed models were used to evaluate the effect of G&C compared to placebo on fecal microbial alpha and beta diversity, seven phyla, and 137 genera. -

Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella Bryantii B14
proteomes Article Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella bryantii B14 Andrej Trautmann 1, Lena Schleicher 2, Simon Deusch 1 , Jochem Gätgens 3 , Julia Steuber 2 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany; [email protected] (A.T.); [email protected] (S.D.) 2 Institute of Biology, University of Hohenheim, 70599 Stuttgart, Germany; [email protected] (L.S.); [email protected] (J.S.) 3 Institute of Bio- and Geosciences, IBG-1: Biotechnology, Forschungszentrum Jülich, 52425 Jülich, Germany; [email protected] * Correspondence: [email protected] Received: 22 September 2020; Accepted: 13 October 2020; Published: 16 October 2020 Abstract: Short-chain fatty acids (SCFAs) are bacterial products that are known to be used as energy sources in eukaryotic hosts, whereas their role in the metabolism of intestinal microbes is rarely explored. In the present study, acetic, propionic, butyric, isobutyric, valeric, and isovaleric acid, respectively, were added to a newly defined medium containing Prevotella bryantii B14 cells. After 8 h and 24 h, optical density, pH and SCFA concentrations were measured. Long-chain fatty acid (LCFA) profiles of the bacterial cells were analyzed via gas chromatography-time of flight-mass spectrometry (GC-ToF MS) and proteins were quantified using a mass spectrometry-based, label-free approach. Cultures supplemented with single SCFAs revealed different growth behavior. Structural features of the respective SCFAs were identified in the LCFA profiles, which suggests incorporation into the bacterial membranes. The proteomes of cultures supplemented with acetic and valeric acid differed by an increased abundance of outer membrane proteins. -

Predictions and Computational Analysis of Novel Chromosomal Type II Toxin Antitoxin Systems in the Human Oral Microbiome
American University in Cairo AUC Knowledge Fountain Theses and Dissertations 6-1-2019 Predictions and Computational Analysis of Novel Chromosomal Type II Toxin Antitoxin Systems in the Human Oral Microbiome Ashraf Abd-el-Raouf Bazan Follow this and additional works at: https://fount.aucegypt.edu/etds Recommended Citation APA Citation Bazan, A. (2019).Predictions and Computational Analysis of Novel Chromosomal Type II Toxin Antitoxin Systems in the Human Oral Microbiome [Master’s thesis, the American University in Cairo]. AUC Knowledge Fountain. https://fount.aucegypt.edu/etds/770 MLA Citation Bazan, Ashraf Abd-el-Raouf. Predictions and Computational Analysis of Novel Chromosomal Type II Toxin Antitoxin Systems in the Human Oral Microbiome. 2019. American University in Cairo, Master's thesis. AUC Knowledge Fountain. https://fount.aucegypt.edu/etds/770 This Thesis is brought to you for free and open access by AUC Knowledge Fountain. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of AUC Knowledge Fountain. For more information, please contact [email protected]. School of Science and Engineering PREDICTION AND COMPUTATIONAL ANALYSIS OF NOVEL CHROMOSOMAL TYPE II TOXIN ANTITOXIN SYSTEMS IN THE HUMAN ORAL MICROBIOME A Thesis Submitted to The Biotechnology Master’s Program In partial fulfillment of the requirements for the degree of Master of Science in Biotechnology By Ashraf A. Bazan BSc in Pharmaceutical Sciences and Drug Design Faculty of Pharmacy, Ain Shams University Under the supervision of Dr. Ahmed Abdellatif, MD, PhD Assistant Professor Biology Department American University in Cairo Dr. Tamer Salem, PhD Dr. Heba Abostate, MD, PhD Professor of Molecular and Cell Biology Assistant Professor of Microbiology Biomedical Science Program, Microbiology and Immunology Department University of Science and Technology at Faculty of Pharmacy Zewail City Egyptian Russian University May/2019 THESIS DEFENSE Student Full Name: ___ Ashraf A. -

Characterizing the Cattle Gut Microbiome in Farms with a High and Low Prevalence of Shiga Toxin-Producing Escherichia Coli
microorganisms Article Characterizing the Cattle Gut Microbiome in Farms with a High and Low Prevalence of Shiga Toxin Producing Escherichia coli Karla Vasco 1 , Brian Nohomovich 1, Pallavi Singh 1,† , Cristina Venegas-Vargas 2,‡, Rebekah E. Mosci 1, Steven Rust 3, Paul Bartlett 2, Bo Norby 2, Daniel Grooms 2,§, Lixin Zhang 1,4 and Shannon D. Manning 1,* 1 Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI 48824, USA; [email protected] (K.V.); [email protected] (B.N.); [email protected] (P.S.); [email protected] (R.E.M.); [email protected] (L.Z.) 2 Department of Large Animal Clinical Sciences, College Veterinary Medicine, Michigan State University, East Lansing, MI 48824, USA; [email protected] (C.V.-V.); [email protected] (P.B.); [email protected] (B.N.); [email protected] (D.G.) 3 Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA; [email protected] 4 Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI 48824, USA * Correspondence: [email protected] † Department of Biological Sciences, Northern Illinois University, DeKalb, IL 60115, USA. ‡ Zoetis Inc., Kalamazoo, MI 49007, USA. § College of Veterinary Medicine, Iowa State University, Ames, IA 50011, USA. Abstract: Cattle are the main reservoirs of Shiga toxin producing Escherichia coli (STEC), a major food- borne pathogen associated with acute enteric disease and hemolytic–uremic syndrome in humans. A Citation: Vasco, K.; Nohomovich, B.; total of 397 beef and dairy cattle from 5 farms were included in this study, of which 660 samples were Singh, P.; Venegas-Vargas, C.; Mosci, collected for 16S rRNA gene sequencing. -

Response of Fecal Bacterial Flora to the Exposure of Fumonisin B1 in BALB/C Mice
toxins Article Response of Fecal Bacterial Flora to the Exposure of Fumonisin B1 in BALB/c Mice Fan Zhang, Zhiwei Chen, Lin Jiang, Zihan Chen and Hua Sun * State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, 1# Xiannongtan Street, Xicheng District, Beijing 100050, China; [email protected] (F.Z.); [email protected] (Z.C.); [email protected] (L.J.); [email protected] (Z.C.) * Correspondence: [email protected] Abstract: Fumonisins are a kind of mycotoxin that has harmful influence on the health of humans and animals. Although some research studies associated with fumonisins have been reported, the regulatory limits of fumonisins are imperfect, and the effects of fumonisins on fecal bacterial flora of mice have not been suggested. In this study, in order to investigate the effects of fumonisin B1 (FB1) on fecal bacterial flora, BALB/c mice were randomly divided into seven groups, which were fed intragastrically with 0 mg/kg, 0.018 mg/kg, 0.054 mg/kg, 0.162 mg/kg, 0.486 mg/kg, 1.458 mg/kg and 4.374 mg/kg of FB1 solutions, once a day for 8 weeks. Subsequently, feces were collected for analysis of microflora. The V3-V4 16S rRNA of fecal bacterial flora was sequenced using the Illumina MiSeq platform. The results revealed that fecal bacterial flora of mice treated with FB1 presented high diversity. Additionally, the composition of fecal bacterial flora of FB1 exposure groups showed marked differences from that of the control group, especially for the genus types including Alloprevotella, Prevotellaceae_NK3B31_group, Rikenellaceae_RC9_gut_group, Parabacteroides and phylum types including Cyanobacteria. -

Supplemental Material
Supplemental Material: Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules Authors: Baker, J.L.1,*, Morton, J.T.2., Dinis, M.3, Alverez, R.3, Tran, N.C.3, Knight, R.4,5,6,7, Edlund, A.1,5,* 1 Genomic Medicine Group J. Craig Venter Institute 4120 Capricorn Lane La Jolla, CA 92037 2Systems Biology Group Flatiron Institute 162 5th Avenue New York, NY 10010 3Section of Pediatric Dentistry UCLA School of Dentistry 10833 Le Conte Ave. Los Angeles, CA 90095-1668 4Center for Microbiome Innovation University of California at San Diego La Jolla, CA 92023 5Department of Pediatrics University of California at San Diego La Jolla, CA 92023 6Department of Computer Science and Engineering University of California at San Diego 9500 Gilman Drive La Jolla, CA 92093 7Department of Bioengineering University of California at San Diego 9500 Gilman Drive La Jolla, CA 92093 *Corresponding AutHors: JLB: [email protected], AE: [email protected] ORCIDs: JLB: 0000-0001-5378-322X, AE: 0000-0002-3394-4804 SUPPLEMENTAL METHODS Study Design. Subjects were included in tHe study if tHe subject was 3 years old or older, in good general HealtH according to a medical History and clinical judgment of tHe clinical investigator, and Had at least 12 teetH. Subjects were excluded from tHe study if tHey Had generalized rampant dental caries, cHronic systemic disease, or medical conditions tHat would influence tHe ability to participate in tHe proposed study (i.e., cancer treatment, HIV, rHeumatic conditions, History of oral candidiasis). Subjects were also excluded it tHey Had open sores or ulceration in tHe moutH, radiation tHerapy to tHe Head and neck region of tHe body, significantly reduced saliva production or Had been treated by anti-inflammatory or antibiotic tHerapy in tHe past 6 montHs.