Xeroderma Pigmentosum Syndrome: a New Model to Study the Role Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elio™ Plasma Complete
™ elio plasma complete About PGDx elioTM plasma complete PGDx elio™ plasma complete is an end-to-end kitted liquid biopsy solution that analyzes circulating tumor DNA for genetic alterations in cancer, eliminating the need for an invasive biopsy or tumor tissue. Designed to be used across the globe on the PGDx elio™ testing platform, PGDx elio plasma complete also includes automated bioinformatics ensuring consistent, high-quality results. What does PGDx elioTM mean? Assay Specifications Empowering Local PARAMETER DETAILS Insight for Oncology Panel Size 2.1MB 521 genes for SNV & Indels 38 genes for amplifications 21 genes for translocations Panel Content and Variant Type bMSI bTMB (Muts/Mb) LOH status Sample requirement plasma ctDNA DNA input requirement 25ng recommended, 10ng minimum End-to-end Kitted 521 Genes From a Single Solution Sample Pass Rate 97.4% overall pass rate (227/233) Sample Sequencing platform/flowcell NovaSeq 6000/S2 flow cell Sequence run 2 x 150 bp Cases per sequencing run 16 (no external control required) Turn-key Developed Under Workflow Manual and Automated Available Bioinformatics Design Control Pipeline Average total coverage ~20,000x Performance Specifications PRODUCT FEATURES Variant Reportable Analytical Analytical Range Sensitivity Specificity (LOD95) Actionable • Plasma analysis for pan-cancer solid ≥ 0.1% VAF 0.40% VAF 100% SNVs/Indels tumor biomarker testing and discovery • 500+ gene kitted assay developed under Non-actionable ≥ 0.5% VAF 1.16% VAF 99.9% Design Control SNVs/Indels • Comprehensive coverage of biomarkers, All clinically relevant targets, cancer ≥ 3 fusion reads 0.33% VAF 100% Translocations signaling pathways and DNA damage repair pathways All ≥ 1.15-fold 1.32-fold 100% • Large panel size supports TMB and LOH Amplifications For Research Use Only. -

Open Full Page
CCR PEDIATRIC ONCOLOGY SERIES CCR Pediatric Oncology Series Recommendations for Childhood Cancer Screening and Surveillance in DNA Repair Disorders Michael F. Walsh1, Vivian Y. Chang2, Wendy K. Kohlmann3, Hamish S. Scott4, Christopher Cunniff5, Franck Bourdeaut6, Jan J. Molenaar7, Christopher C. Porter8, John T. Sandlund9, Sharon E. Plon10, Lisa L. Wang10, and Sharon A. Savage11 Abstract DNA repair syndromes are heterogeneous disorders caused by around the world to discuss and develop cancer surveillance pathogenic variants in genes encoding proteins key in DNA guidelines for children with cancer-prone disorders. Herein, replication and/or the cellular response to DNA damage. The we focus on the more common of the rare DNA repair dis- majority of these syndromes are inherited in an autosomal- orders: ataxia telangiectasia, Bloom syndrome, Fanconi ane- recessive manner, but autosomal-dominant and X-linked reces- mia, dyskeratosis congenita, Nijmegen breakage syndrome, sive disorders also exist. The clinical features of patients with DNA Rothmund–Thomson syndrome, and Xeroderma pigmento- repair syndromes are highly varied and dependent on the under- sum. Dedicated syndrome registries and a combination of lying genetic cause. Notably, all patients have elevated risks of basic science and clinical research have led to important in- syndrome-associated cancers, and many of these cancers present sights into the underlying biology of these disorders. Given the in childhood. Although it is clear that the risk of cancer is rarity of these disorders, it is recommended that centralized increased, there are limited data defining the true incidence of centers of excellence be involved directly or through consulta- cancer and almost no evidence-based approaches to cancer tion in caring for patients with heritable DNA repair syn- surveillance in patients with DNA repair disorders. -

EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies
Focusing on Personalised Medicine EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies Extended carrier screening is an important tool for prospective parents to help them determine their risk of having a child affected with a heritable disease. In many cases, parents aren’t aware they are carriers and have no family history due to the rarity of some diseases in the general population. What is covered by the screening? Genomics For Life offers a comprehensive Extended Carrier Screening test, providing prospective parents with the information they require when planning their pregnancy. Extended Carrier Screening has been shown to detect carriers who would not have been considered candidates for traditional risk- based screening. With a simple mouth swab collection, we are able to test for over 419 genes associated with inherited diseases, including Fragile X Syndrome, Cystic Fibrosis and Spinal Muscular Atrophy. The assay has been developed in conjunction with clinical molecular geneticists, and includes genes listed in the NIH Genetic Test Registry. For a list of genes and disorders covered, please see the reverse of this brochure. If your gene of interest is not covered on our Extended Carrier Screening panel, please contact our friendly team to assist you in finding a gene test panel that suits your needs. Why have Extended Carrier Screening? Extended Carrier Screening prior to pregnancy enables couples to learn about their reproductive risk and consider a complete range of reproductive options, including whether or not to become pregnant, whether to use advanced reproductive technologies, such as preimplantation genetic diagnosis, or to use donor gametes. -

Large XPF-Dependent Deletions Following Misrepair of a DNA Double Strand Break Are Prevented by the RNA:DNA Helicase Senataxin
www.nature.com/scientificreports OPEN Large XPF-dependent deletions following misrepair of a DNA double strand break are prevented Received: 26 October 2017 Accepted: 9 February 2018 by the RNA:DNA helicase Published: xx xx xxxx Senataxin Julien Brustel1, Zuzanna Kozik1, Natalia Gromak2, Velibor Savic3,4 & Steve M. M. Sweet1,5 Deletions and chromosome re-arrangements are common features of cancer cells. We have established a new two-component system reporting on epigenetic silencing or deletion of an actively transcribed gene adjacent to a double-strand break (DSB). Unexpectedly, we fnd that a targeted DSB results in a minority (<10%) misrepair event of kilobase deletions encompassing the DSB site and transcribed gene. Deletions are reduced upon RNaseH1 over-expression and increased after knockdown of the DNA:RNA helicase Senataxin, implicating a role for DNA:RNA hybrids. We further demonstrate that the majority of these large deletions are dependent on the 3′ fap endonuclease XPF. DNA:RNA hybrids were detected by DNA:RNA immunoprecipitation in our system after DSB generation. These hybrids were reduced by RNaseH1 over-expression and increased by Senataxin knock-down, consistent with a role in deletions. Overall, these data are consistent with DNA:RNA hybrid generation at the site of a DSB, mis-processing of which results in genome instability in the form of large deletions. DNA is the target of numerous genotoxic attacks that result in diferent types of damage. DNA double-strand breaks (DSBs) occur at low frequency, compared with single-strand breaks and other forms of DNA damage1, however DSBs pose the risk of translocations and deletions and their repair is therefore essential to cell integrity. -

Disease Reference Book
The Counsyl Foresight™ Carrier Screen 180 Kimball Way | South San Francisco, CA 94080 www.counsyl.com | [email protected] | (888) COUNSYL The Counsyl Foresight Carrier Screen - Disease Reference Book 11-beta-hydroxylase-deficient Congenital Adrenal Hyperplasia .................................................................................................................................................................................... 8 21-hydroxylase-deficient Congenital Adrenal Hyperplasia ...........................................................................................................................................................................................10 6-pyruvoyl-tetrahydropterin Synthase Deficiency ..........................................................................................................................................................................................................12 ABCC8-related Hyperinsulinism........................................................................................................................................................................................................................................ 14 Adenosine Deaminase Deficiency .................................................................................................................................................................................................................................... 16 Alpha Thalassemia............................................................................................................................................................................................................................................................. -

The Genetic Basis of Dupuytren's Disease Gloria Sue Yale School of Medicine, [email protected]
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2014 The Genetic Basis Of Dupuytren's Disease Gloria Sue Yale School of Medicine, [email protected] Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Sue, Gloria, "The Genetic Basis Of Dupuytren's Disease" (2014). Yale Medicine Thesis Digital Library. 1926. http://elischolar.library.yale.edu/ymtdl/1926 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. The Genetic Basis of Dupuytren’s Disease A Thesis Submitted to the Yale University School of Medicine In Partial Fulfillment of the Requirements for the Degree of Doctor of Medicine by Gloria R. Sue 2014 THE GENETIC BASIS OF DUPUYTREN’S DISEASE. Gloria R. Sue, Deepak Narayan. Section of Plastic and Reconstructive Surgery, Department of Surgery, Yale University School of Medicine, New Haven, CT. Dupuytren’s disease is a common heritable connective tissue disorder of poorly understood etiology. It is thought that oxidative stress pathways may play a critical role in the development of Dupuytren’s disease, given the various disease associations that have been observed. We sought to sequence the mitochondrial and nuclear genomes of patients affected with Dupuytren’s disease using next-generation sequencing technology to potentially identify genes of potential pathogenetic interest. -

Emaciation, Congestive Heart Failure, and Systemic Amyloidosis In
Case Report Emaciation, Congestive Heart Failure, and Systemic Amyloidosis in Severe Recessive Dystrophic Epidermolysis Bullosa: Possible Internal Complications Due to Skin-Derived Inflammatory Cytokines Derived from the Injured Skin Yoshiaki Matsushima y , Kento Mizutani y, Hiroyuki Goto y, Takehisa Nakanishi, Makoto Kondo, Koji Habe, Kenichi Isoda, Hitoshi Mizutani and Keiichi Yamanaka * Department of Dermatology, Mie University Graduate School of Medicine, Tsu, Mie 514-8507, Japan; [email protected] (Y.M.); [email protected] (K.M.); [email protected] (H.G.); [email protected] (T.N.); [email protected] (M.K.); [email protected] (K.H.); [email protected] (K.I.); [email protected] (H.M.) * Correspondence: [email protected]; Tel.: +81-59-231-5025; Fax: +81-59-231-5206 These authors contributed equally to this work. y Received: 2 August 2020; Accepted: 7 September 2020; Published: 14 September 2020 Abstract: Inherited epidermolysis bullosa (EB) is a rare genetic skin disorder characterized by epithelial tissue fragility. Recessive dystrophic epidermolysis bullosa (RDEB) is the most severe form, characterized by the presence of blisters, erosion, and ulcer formation, leading to scarring and contraction of the limbs. RDEB is also associated with extra-cutaneous complications, including emaciation, congestive heart failure, and systemic amyloidosis. The main cause of these clinical complications is unknown; however, we hypothesized that they are caused by elevated circulating inflammatory cytokines overproduced by injured keratinocytes. We addressed this phenomenon using keratin-14 driven, caspase-1 overexpressing, transgenic (KCASP1Tg) mice in which injured keratinocytes release high levels of IL-1α and β. -
Blueprint Genetics Ectodermal Dysplasia Panel
Ectodermal Dysplasia Panel Test code: DE0401 Is a 25 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of ectodermal dysplasia (hidrotic or hypohidrotic) or Ellis-van Creveld syndrome. About Ectodermal Dysplasia Ectodermal Dysplasia (ED) is a group of closely related conditions of which more than 150 different syndromes have been identified. EDs affects the development or function of teeth, hair, nails and sweat glands. ED may present as isolated or as part of a syndromic disease and is commonly subtyped according to sweating ability. The clinical features of the X-linked and autosomal forms of hypohidrotic ectodermal dysplasia (HED) can be indistinguishable and many of the involved genes may lead to phenotypically distinct outcomes depending on number of defective alleles. The most common EDs are hypohidrotic ED and hydrotic ED. X-linked hypohidrotic ectodermal dysplasia (HED) is caused by EDA mutations and explain 75%-95% of familial HED and 50% of sporadic cases. HED is characterized by three cardinal features: hypotrichosis (sparse, slow-growing hair and sparse/missing eyebrows), reduced sweating and hypodontia (absence or small teeth). Reduced sweating poses risk for episodes of hyperthermia. Female carriers may have some degree of hypodontia and mild hypotrichosis. Isolated dental phenotypes have also been described. Mutations in WNT10A have been reported in up to 9% of individuals with HED and in 25% of individuals with HED who do not have defective EDA. Approximately 50% of individuals with heterozygous WNT10A mutation have HED and the most consistent clinical feature is severe oligodontia of permanent teeth. -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Epidermolysis Bullosa (EB)
OR Preparation for Patients with Epidermolysis Bullosa (EB) Background: Patients with EB have a mutation in their keratin or collagen genes. As a result the skin is not properly anchored and mere touch can cause the skin to slough or blister. Many different subtypes have been identified but the most common variant we see in our patient population is recessive dystrophic EB. The children appear as burn patients and are frequently wrapped in total body burn dressings. NO ADHESIVES MAY BE USED IN THESE PATIENTS. You will note the following in these children. Airway: the tissues of the lining of the mouth, tongue and esophagus are affected making eating difficult. Mouth opening is SEVERELY LIMITED and these patients are always considered difficult airways. FOI is the preferred method to intubate since we try to minimize contact with the mucosa. Digits: continued skin slough and scarring results in absence of fingernails and the fingers often fuse together. Cardiac: Over time, some children develop a cardiomyopathy. Anemia is common as is continued infections, so these children often appear to be in a high-output state. Renal: Many children develop renal failure over time. Hemotologic: Continued bleeding from wounds and poor nutrition causes anemia. Infections: These children are often colonized with MRSA in their wounds and many act as though in low-grade sepsis. You will see tachycardia and anesthetic resistance. IV access: Difficult though not as impossible as you would imagine. NO ELASTIC TOURNIQUETS! A hand tourniquet is sufficient. Alcohol wipes are not usually used. A small amount of baby shampoo on moistened gauze dabbed on and off with moist gauze may be used GI tract: Esophageal strictures are common due to sloughing and scarring of the esophagus, so patients coming in for these procedures often cannot handle their oral secretions and are drooling. -
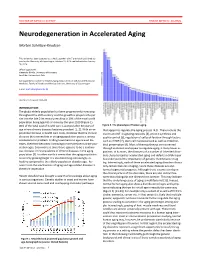
Neurodegeneration in Accelerated Aging
DOCTOR OF MEDICAL SCIENCE DANISH MEDICAL JOURNAL Neurodegeneration in Accelerated Aging Morten Scheibye-Knudsen This review has been accepted as a thesis together with 7 previously published pa- pers by the University of Copenhagen, October 16, 2014 and defended on January 14, 2016 Official opponents: Alexander Bürkle, University of Konstanz Lars Eide, University of Oslo Correspondence: Center for Healthy Aging, Department of Cellular and Molecular Medicine, Faculty of Health and Medical Sciences, University of Copenhagen E-mail: [email protected] Dan Med J 2016;63(11):B5308 INTRODUCTION The global elderly population has been progressively increasing throughout the 20th century and this growth is projected to per- sist into the late 21st century resulting in 20% of the total world population being aged 65 or more by the year 2100 (Figure 1). 80% of the total cost of health care is accrued after 40 years of Figure 2. The phenotype of human aging. age where chronic diseases become prevalent [1, 2]. With an ex- that appear to regulate the aging process [4,5]. These include the ponential increase in health care costs, it follows that the chronic insulin and IGF-1 signaling cascades [4], protein synthesis and diseases that accumulate in an aging population poses a serious quality control [6], regulation of cell proliferation through factors socioeconomic problem. Finding treatments to age related dis- such as mTOR [7], stem cell maintenance 8 as well as mitochon- eases, therefore becomes increasingly more pertinent as the pop- drial preservation [9]. Most of these pathways are conserved ulation ages. Even more so since there appears to be a continu- through evolution and appear to regulate aging in many lower or- ous increase in the prevalence of chronic diseases in the aging ganisms. -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body.