Emaciation, Congestive Heart Failure, and Systemic Amyloidosis In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Epidermolysis Bullosa (EB)
OR Preparation for Patients with Epidermolysis Bullosa (EB) Background: Patients with EB have a mutation in their keratin or collagen genes. As a result the skin is not properly anchored and mere touch can cause the skin to slough or blister. Many different subtypes have been identified but the most common variant we see in our patient population is recessive dystrophic EB. The children appear as burn patients and are frequently wrapped in total body burn dressings. NO ADHESIVES MAY BE USED IN THESE PATIENTS. You will note the following in these children. Airway: the tissues of the lining of the mouth, tongue and esophagus are affected making eating difficult. Mouth opening is SEVERELY LIMITED and these patients are always considered difficult airways. FOI is the preferred method to intubate since we try to minimize contact with the mucosa. Digits: continued skin slough and scarring results in absence of fingernails and the fingers often fuse together. Cardiac: Over time, some children develop a cardiomyopathy. Anemia is common as is continued infections, so these children often appear to be in a high-output state. Renal: Many children develop renal failure over time. Hemotologic: Continued bleeding from wounds and poor nutrition causes anemia. Infections: These children are often colonized with MRSA in their wounds and many act as though in low-grade sepsis. You will see tachycardia and anesthetic resistance. IV access: Difficult though not as impossible as you would imagine. NO ELASTIC TOURNIQUETS! A hand tourniquet is sufficient. Alcohol wipes are not usually used. A small amount of baby shampoo on moistened gauze dabbed on and off with moist gauze may be used GI tract: Esophageal strictures are common due to sloughing and scarring of the esophagus, so patients coming in for these procedures often cannot handle their oral secretions and are drooling. -

Ichthyosis: Case Report in a Colombian Man with Genetic Alterations in ABCA12 and HRNR Genes Ruben D
Arias‑Pérez et al. BMC Med Genomics (2021) 14:140 https://doi.org/10.1186/s12920‑021‑00987‑y CASE REPORT Open Access Ichthyosis: case report in a Colombian man with genetic alterations in ABCA12 and HRNR genes Ruben D. Arias‑Pérez1, Salomón Gallego‑Quintero1, Natalia A. Taborda1 , Jorge E. Restrepo2, Renato Zambrano‑Cruz3 , William Tamayo‑Agudelo3, Patricia Bermúdez4, Constanza Duque4, Ismael Arroyave4, Johanna A. Tejada‑Moreno5, Andrés Villegas‑Lanau5, Alejandro Mejía‑García5, Wildeman Zapata6 , Juan C. Hernandez6* and Gina Cuartas‑Montoya3 Abstract Background: Ichthyosis is a heterogeneous group of diseases caused by genetic disorders related to skin formation. They are characterized by generalized dry skin, scaling, hyperkeratosis and frequently associated with erythroderma. Among its diferent types, harlequin ichthyosis (HI) stands out due to its severity. HI is caused by mutations in the ABCA12 gene, which encodes essential proteins in epidermal lipid transport, and it helps maintain the homeostasis of the stratum corneum of the epidermis. However, due to the wide spectrum of genetic alterations that can cause ichthyosis, holistic medical care, and genetic studies are required to improve the diagnosis and outcomes of these diseases. Case presentation: Here, we presented the case of a 19 years old male patient who was a premature infant and exhibited clinical features consistent with HI, including bright yellow hyperkeratotic plates with erythematous fssures that covered his entire body like a collodion baby. Currently, he exhibited erythroderma, photosensitivity, ectropion, auricular pavilion alterations, and musculoskeletal disorders, such as equinovarus feet, fngers, hands, and hypoplastic feet with contractures in fexion and marked difculty in fne motor skills. -

Blueprint Genetics Epidermolysis Bullosa Panel
Epidermolysis Bullosa Panel Test code: DE0301 Is a 26 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of congenital epidermolysis bullosa. About Epidermolysis Bullosa Epidermolysis bullosa (EB) is a group of inherited diseases that are characterised by blistering lesions on the skin and mucous membranes, most commonly appearing at sites of friction and minor trauma such as the feet and hands. In some subtypes, blisters may also occur on internal organs, such as the oesophagus, stomach and respiratory tract, without any apparent friction. There are 4 major types of EB based on different sites of blister formation within the skin structure: Epidermolysis bullosa simplex (EBS), Junctional epidermolysis bullosa (JEB), Dystrophic epidermolysis bullosa (DEB), and Kindler syndrome (KS). EBS is usually characterized by skin fragility and rarely mucosal epithelia that results in non-scarring blisters caused by mild or no trauma. The four most common subtypes of EBS are: 1) localized EBS (EBS-loc; also known as Weber-Cockayne type), 2) Dowling-Meara type EBS (EBS-DM), 3) other generalized EBS(EBS, gen-nonDM; also known as Koebner type) and 4) EBS-with mottled pigmentation (EBS-MP). Skin biopsy from fresh blister is considered mandatory for diagnostics of generalized forms of EBS. The prevalence of EBS is is estimated to be 1:30,000 - 50,000. EBS-loc is the most prevalent, EBS- DM and EBS-gen-nonDM are rare, and EBS-MP is even rarer. Penetrance is 100% for known KRT5 and KRT14 mutations. Location of the mutations within functional domains of KRT5and KRT14 has shown to predict EBS phenotype. -
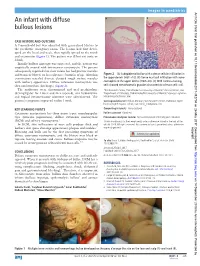
An Infant with Diffuse Bullous Lesions
Images in paediatrics Arch Dis Child: first published as 10.1136/archdischild-2017-312704 on 26 May 2017. Downloaded from An infant with diffuse bullous lesions CASE HISTORY, AND OUTCOME A 7-month-old boy was admitted with generalised blisters to the paediatric emergency room. The lesions had first devel- oped on the head and neck, then rapidly spread to the trunk and extremities (figure 1). The patient was ill but not toxic or febrile. Initially bullous impetigo was suspected, and the patient was empirically treated with intravenous vancomycin. The parents subsequently reported that their infant has had pruritic macules and transient blisters on his scalp since 5 months of age. Also skin Figure 2 (A) Subepidermal bullae with a dense cellular infiltration in examination revealed discrete elevated rough surface macules the upper dermis (H&E ×10). (B) Dense mast cell infiltration with some with leathery appearance. Diffuse cutaneous mastocytosis was eosinophils in the upper dermis (H&E×20). (C) With Giemsa staining, then confirmed on skin biopsy (figure 2). cells showed metachromatic granules characteristic of mast cells ×40. The antibiotics were discontinued and oral prednisolone 1Skin Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran (0.5 mg/kg/day for 1 week and then tapered), oral hydroxyzine 2Department of Pathology, Shahid Beheshti University of Medical Sciences, Loghman- and topical betamethasone ointment were administered. The Hakim Hospital, Tehran, Iran patient’s symptoms improved within 1 week. Correspondence to Dr Nikoo Mozafari, Skin Research Center, Shohada e Tajrish hospital, Tajrish Square, Tehran, Iran; nikoo_ md@ yahoo. com KEY LEARNING POINTS Competing interests None declared. -

How to Deal with Skin Biopsy in an Infant with Blisters?
Review How to Deal with Skin Biopsy in an Infant with Blisters? Stéphanie Leclerc-Mercier Reference Center for Genodermatoses (MAGEC Center), Department of Pathology, Necker-Enfants Malades Hospital, Paris Centre University, 75015 Paris, France; [email protected] Abstract: The onset of blisters in a neonate or an infant is often a source of great concern for both parents and physicians. A blistering rash can reveal a wide range of diseases with various backgrounds (infectious, genetic, autoimmune, drug-related, traumatic, etc.), so the challenge for the dermatologist and the pediatrician is to quickly determine the etiology, between benign causes and life-threatening disorders, for a better management of the patient. Clinical presentation can provide orientation for the diagnosis, but skin biopsy is often necessary in determining the cause of blister formations. In this article, we will provide information on the skin biopsy technique and discuss the clinical orientation in the case of a neonate or infant with a blistering eruption, with a focus on the histology for each etiology. Keywords: blistering eruption; infant; skin biopsy; genodermatosis; SSSS; hereditary epidermolysis bul- losa; keratinopathic ichthyosis; incontinentia pigmenti; mastocytosis; auto-immune blistering diseases 1. Introduction The onset of blisters in a neonate or an infant (<2 years old) is a source of great concern for both parents and physicians. Therefore, a precise diagnosis, between benign causes and Citation: Leclerc-Mercier, S. How to life-threatening disorders, is quickly needed for the best management of the baby. Deal with Skin Biopsy in an Infant Several diseases with various backgrounds (infectious, genetic, autoimmune, drug- with Blisters? Dermatopathology 2021, related, traumatic, etc.) can lead to a blistering eruption. -

Table I. Genodermatoses with Known Gene Defects 92 Pulkkinen
92 Pulkkinen, Ringpfeil, and Uitto JAM ACAD DERMATOL JULY 2002 Table I. Genodermatoses with known gene defects Reference Disease Mutated gene* Affected protein/function No.† Epidermal fragility disorders DEB COL7A1 Type VII collagen 6 Junctional EB LAMA3, LAMB3, ␣3, 3, and ␥2 chains of laminin 5, 6 LAMC2, COL17A1 type XVII collagen EB with pyloric atresia ITGA6, ITGB4 ␣64 Integrin 6 EB with muscular dystrophy PLEC1 Plectin 6 EB simplex KRT5, KRT14 Keratins 5 and 14 46 Ectodermal dysplasia with skin fragility PKP1 Plakophilin 1 47 Hailey-Hailey disease ATP2C1 ATP-dependent calcium transporter 13 Keratinization disorders Epidermolytic hyperkeratosis KRT1, KRT10 Keratins 1 and 10 46 Ichthyosis hystrix KRT1 Keratin 1 48 Epidermolytic PPK KRT9 Keratin 9 46 Nonepidermolytic PPK KRT1, KRT16 Keratins 1 and 16 46 Ichthyosis bullosa of Siemens KRT2e Keratin 2e 46 Pachyonychia congenita, types 1 and 2 KRT6a, KRT6b, KRT16, Keratins 6a, 6b, 16, and 17 46 KRT17 White sponge naevus KRT4, KRT13 Keratins 4 and 13 46 X-linked recessive ichthyosis STS Steroid sulfatase 49 Lamellar ichthyosis TGM1 Transglutaminase 1 50 Mutilating keratoderma with ichthyosis LOR Loricrin 10 Vohwinkel’s syndrome GJB2 Connexin 26 12 PPK with deafness GJB2 Connexin 26 12 Erythrokeratodermia variabilis GJB3, GJB4 Connexins 31 and 30.3 12 Darier disease ATP2A2 ATP-dependent calcium 14 transporter Striate PPK DSP, DSG1 Desmoplakin, desmoglein 1 51, 52 Conradi-Hu¨nermann-Happle syndrome EBP Delta 8-delta 7 sterol isomerase 53 (emopamil binding protein) Mal de Meleda ARS SLURP-1 -

Clinical Practice Guidelines for Laboratory Diagnosis of Epidermolysis Bullosa 1 2 3 4 5 6 7,8 C
BJD GUIDELINE British Journal of Dermatology Clinical practice guidelines for laboratory diagnosis of epidermolysis bullosa 1 2 3 4 5 6 7,8 C. Has iD , L. Liu, M.C. Bolling, A.V. Charlesworth, M. El Hachem iD , M.J. Escamez, I. Fuentes, 1 9 10 11 12 S. Buchel,€ R. Hiremagalore, G. Pohla-Gubo, P.C. van den Akker iD , K. Wertheim-Tysarowska and G. Zambruno5 1Department of Dermatology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany 2Viapath, St Thomas’ Hospital, London, U.K. Departments of 3Dermatology and 11Genetics, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands 4Centre de Reference des Maladies Rares de la Peau et des Muqueuses d’Origine Genetique, L’Archet H^opital, Nice, France 5Dermatology Unit, Bambino Gesu Children’s Hospital, IRCCS, Rome, Italy 6Bioengineering Department at Universidad Carlos III de Madrid (UC3M), Regenerative Medicine Unit at CIEMAT – U714 CIBER on Rare Diseases (ISCIII), Instituto de Investigacion Sanitaria Fundacion Jimenez Diaz (IISFJD), Madrid, Spain 7Fundacion DEBRA Chile, Santiago, Chile 8Centro de Genetica y Genomica, Facultad de Medicina, Clınica Alemana Universidad del Desarrollo, Santiago, Chile 9Adjunct Faculty, Centre for Human Genetics and Department of Dermatology and Pediatrics, Manipal Hospital, Bengaluru, India 10EB House Austria, Department of Dermatology, University Hospital of the Paracelsus Medical University, Salzburg, Austria 12Department of Medical Genetics, Institute of Mother and Child, Warsaw, Poland Correspondence Cristina Has. 1.0 Purpose and scope E-mail: [email protected] The overall objective of this guideline is to provide the user Accepted for publication with information on the laboratory diagnosis of inherited epi- 9 May 2019 dermolysis bullosa (EB) to improve outcomes (Table 1). -

The Clinical Spectrum of Congenital
Acta Derm Venereol 2003, Suppl. 213: 34–47 The Clinical Spectrum of Congenital Ichthyosis in Sweden: A Review of 127 Cases ANDERS VAHLQUIST1, AGNETA GÅNEMO1, MARITTA PIGG2, MARIE VIRTANEN1 AND PER WESTERMARK3 Departments of 1Dermatology, 2Clinical Genetics and 3Pathology, University Hospital, Uppsala, Sweden Congenital ichthyosis comprises a rare group of usual- microscopy, HI: Harlequin ichthyosis, IFAP: ichthyosis follicularis, ly monogenetic diseases that present at birth as a collo- alopecia and photofobia, LI-TGM: lamellar ichthyosis with dion phenotype or as variable degrees of ichthyosiform transglutaminase 1 gene mutations, LI/CIE: lamellar ichthyosis and/ or congenital ichthyosiform erythroderma, KID: keratitis, ichthyo- erythroderma, with or without superficial blisters. De- sis and deafness, K: keratin, KLICK: keratosis linearis, ichthyosis pending on which gene mutation causes the disease, the congenita and keratoderma, SLS: Sjögren-Larsson syndrome, Tgase skin problems later in life may range from a severe 1 – transglutaminase 1 protein, XRI: X-linked recessive ichthyosis. lamellar or bullous ichthyosis to mild or only focally expressed hyperkeratotic lesions. It is obviously impor- tant, but sometimes painstakingly difficult, to make a INTRODUCTION correct diagnosis already in infancy. Fortunately, re- cent advances in our understanding of the molecular Congenital ichthyosis (CI) encompasses a large group of genetics of ichthyosis have led to several new diagnos- mostly monogenetic disorders of keratinization present- tic tools that are continuously being updated. Based on ing at birth as widespread hyperkeratosis and scaling of the integument, and sometimes associated with erythro- Downloaded By: [Akademiska Sjukhuset] At: 12:48 5 October 2007 this development, and on our own 5 years of experi- ence in a national genodermatosis centre, we describe derma and skin erosions. -

Inside Out: Regenerative Medicine for Recessive Dystrophic Epidermolysis Bullosa
Review nature publishing group Inside out: regenerative medicine for recessive dystrophic epidermolysis bullosa Michael Vanden Oever1, Kirk Twaroski2, Mark J. Osborn1, John E. Wagner1 and Jakub Tolar1 Epidermolysis bullosa is classified as a genodermatosis, an human skin is capable of regenerating injured tissue and inherited genetic skin disorder that results in severe, chronic maintaining a homeostatic state. However, in the case of skin blistering with painful and life-threatening complications. RDEB, the supporting cells near the DEJ are unable to Although there is currently no cure for epidermolysis bullosa, produce functional C7 and generate a normal healing concurrent advances in gene and stem cell therapies are response. Furthermore, chronic wounds such as those found converging toward combinatorial therapies that hold the in patients with RDEB have shown a limited ability to promise of clinically meaningful and lifelong improvement. remodel the extracellular matrix in a productive manner, Recent studies using hematopoietic stem cells and mesench- further limiting the ability for RDEB skin to regenerate itself. ymal stromal/stem cells to treat epidermolysis bullosa have Although many of the signs and symptoms of RDEB are demonstrated the potential for sustained, effective manage- apparent at birth, certain aspects of the disease pathology are ment of the most severe cases. Furthermore, advances in the progressive in nature. Loss of the physical barrier and use of gene therapy and gene-editing techniques, coupled disruption of the immunologic function of the skin leads to with the development of induced pluripotent stem cells from persistent chronic infections, acquired resistance to antibio- patients with epidermolysis bullosa, allow for autologous tics, and can become refractory to interventional therapies. -

Epidermolysis Bullosa Simplex
EPIDERMOLYSIS BULLOSA SIMPLEX 1. Introduction Epidermolysis bullosa simplex is an umbrella term for all forms of EB, where the blister formation develops within the outermost skin layer. The term "simplex" sometimes makes one tempted to believe that it is "simple", that it refers to uncomplicated and simple forms of EB. That's not quite right. It is true that some forms of EB compared with other forms of EB simplex seem less severe, it means a life can have limitations that can be different or perceived as stressful. There are also some forms of EB simplex, which are very rare, but have very serious effects on the lives and well being among the people with more severe forms of EB. It is therefore very important to know the exact diagnosis when EBS is suspected, if you want to adapt to the future course of the disease. Important points in a nutshell Epidermolysis bullosa simplex is the general term for all forms of EB, where blistering takes place within the outer skin/epidermis. EBS is caused by mutations in different genes. "Simplex" is not synonymous with "simple". Page 1 of 2 © A. Diem, B. Sailer: EBS generalized severe, 08/2012 (new classification 2015) Translated by Lynne Hinterbuchner 2. EBS generalized severe EBS generalized severe is caused by mutations in the genes of keratin 5 or 14. It is an autosomal dominant disorder, in most cases the disease is already known in the family. Instances of new cases by so-called spontaneous mutations occur, but for more detailed explanations, see the topic "Genetics”. -

Junctional Epidermolysis Bullosa
Junctional epidermolysis bullosa Description Junctional epidermolysis bullosa (JEB) is a major form of epidermolysis bullosa, a group of genetic conditions that cause the skin to be very fragile and to blister easily. Blisters and areas of skin loss (erosions) form in response to minor injury or friction, such as rubbing or scratching. Researchers classify junctional epidermolysis bullosa into two main types: JEB generalized severe (formerly known as Herlitz JEB) and JEB generalized intermediate (formerly known as non-Herlitz JEB). Although the types differ in severity, their features overlap significantly, and they can be caused by mutations in the same genes. JEB generalized severe is the more serious form of the condition. From birth or early infancy, affected individuals have blistering over large regions of the body. Blistering also affects the mucous membranes, such as the moist lining of the mouth and digestive tract, which can make it difficult to eat and digest food. As a result, many affected children are undernourished and grow slowly. The extensive blistering leads to scarring and the formation of red, bumpy patches called granulation tissue. Granulation tissue bleeds easily and profusely, making affected infants susceptible to serious infections and loss of necessary proteins, minerals, and fluids. Additionally, a buildup of granulation tissue in the airway can lead to a weak, hoarse cry and difficulty breathing. Other complications of JEB generalized severe can include fusion of the fingers and toes, abnormalities of the fingernails and toenails, joint deformities (contractures) that limit movement, hair loss (alopecia), and thinning of the protective outer layer (enamel) of the teeth. -

Genetic Correction of Stem Cells in the Treatment of Inherited Diseases and Focus on Xeroderma Pigmentosum
Int. J. Mol. Sci. 2013, 14, 20019-20036; doi:10.3390/ijms141020019 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Genetic Correction of Stem Cells in the Treatment of Inherited Diseases and Focus on Xeroderma Pigmentosum Sophie Rouanet 1,†, Emilie Warrick 2,†, Yannick Gache 1, Sabine Scarzello 1, Marie-Françoise Avril 3, Françoise Bernerd 2 and Thierry Magnaldo 1,* 1 Genetics and Physiopathology of Epithelial Cancers, INSERM U 1081-CNRS UMR 7284-UNS, Institute for Research on Cancer and Aging, Nice, Medical school, 28 Avenue de Valombrose, 06107 Nice Cedex 2, France; E-Mails: [email protected] (S.R.); [email protected] (Y.G.); [email protected] (S.S.) 2 L’Oréal Research and Innovation, 92217 Clichy, France; E-Mails: [email protected] (E.W.); [email protected] (F.B.) 3 Hopital Cochin, Pavillon Tarnier, APHP, Université Paris 6, 75006 Paris, France; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-0-4-9337-7670; Fax: +33-0-4-9337-7786. Received: 29 July 2013; in revised form: 11 September 2013 / Accepted: 17 September 2013 / Published: 9 October 2013 Abstract: Somatic stem cells ensure tissue renewal along life and healing of injuries. Their safe isolation, genetic manipulation ex vivo and reinfusion in patients suffering from life threatening immune deficiencies (for example, severe combined immunodeficiency (SCID)) have demonstrated the efficacy of ex vivo gene therapy. Similarly, adult epidermal stem cells have the capacity to renew epidermis, the fully differentiated, protective envelope of our body.