Rho Kinase Regulates Schwann Cell Myelination and Formation of Associated Axonal Domains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Schwann Cells (Cell Culture/Laminin) SETH PORTER*T, Luis GLASER*T, and RICHARD P
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 7768-7772, November 1987 Neurobiology Release of autocrine growth factor by primary and immortalized Schwann cells (cell culture/laminin) SETH PORTER*t, Luis GLASER*t, AND RICHARD P. BUNGEt Departments of *Biological Chemistry and tAnatomy and Neurobiology, Washington University School of Medicine, St. Louis, MO 63110; and tDepartments of Biology and Biochemistry, University of Miami, Coral Gables, FL 33156 Communicated by Gerald D. Fischbach, July 20, 1987 ABSTRACT Schwann cells derived from neonatal rat proliferation. Because Schwann cells secrete laminin and sciatic nerve are quiescent in culture unless treated with specific form a basement membrane at an initial stage of neuronal mitogens. The use of glial growth factor (GGF) and forskolin ensheathment (21), it is important to establish the role of has been found to be an effective method for stimulating these components in the control of proliferation and differ- proliferation of Schwann cells on a poly(L-lysine) substratum entiation. while maintaining their ability to myelinate axons in vitro. We We report that repetitive passaging of Schwann cells find that repetitive passaging of Schwann cells with GGF and derived from neonatal rat can result in a population of forskolin results in the loss of normal growth control; the cells immortalized cells that, depending upon their duration in are able to proliferate without added mitogens. The immor- culture, display all or most ofthe functional characteristics of talized cells grow continuously in the absence of added growth a primary Schwann cell. It was found that both immortalized factor and in the presence or absence of serum yet continue to and primary Schwann cells secrete growth-promoting activ- express distinctive Schwann cell-surface antigens. -

Clustering of Na+ Channels and Node of Ranvier Formation in Remyelinating Axons
The Journal of Neuroscience, January 1995, 15(l): 492503 Clustering of Na+ Channels and Node of Ranvier Formation in Remyelinating Axons Sanja Dugandgija-NovakoviC,’ Adam G. Koszowski,2 S. Rock Levinson,2 and Peter Shragerl ‘Department of Physiology, University of Rochester Medical Center, Rochester, New York 14642 and 2Department of Physiology, University of Colorado Health Sciences Center, Denver, Colorado 80262 Polyclonal antibodies were raised against a well conserved nodal regions(Black et al., 1990). The density of Na+ channels, region of the vertebrate Na+ channel and were affinity pu- in particular, is about 25 times higher at nodesof Ranvier than rified for use in immunocytochemistry. Focal demyelination at internodal sites (Shrager, 1989). There has been vigorous of rat sciatic axons was initiated by an intraneural injection debate over the mechanism of Na+ channel clustering during of lysolecithin and Na+ channel clustering was followed at myelination, particularly with respect to the role of Schwann several stages of myelin removal and repair. At 1 week post- cells, and studies have included both developing nerve and injection axons contained long, fully demyelinated regions. pathological conditions (Ellisman, 1979; Rosenbluth, 1979; Ro- Na+ channel clusters appeared only at heminodes forming senbluth and Blakemore, 1984; Le Beau et al., 1987; England the borders of these zones, and at widely spaced isolated et al., 1990, 1991; Joe and Angelides, 1992, 1993).There remain sites that may represent former nodes of Ranvier. Over the many interesting questions, particularly regarding remodeling next few days proliferating Schwann cells adhered to axons that occurs following myelin disruption. When axons are de- and began to extend processes. -

Schwann Cell Processes Guide Regeneration of Peripheral Axons
Neuron, Vol. 14, 125-132, January, 1995, Copyright © 1995 by Cell Press Schwann Cell Processes Guide Regeneration of Peripheral Axons Young-Jin Son and Wesley J. Thompson come immunoreactive (Astrow et al., 1994). There is a Center for Developmental Biology similar up-regulation of immunoreactivity in Schwann cells and Department of Zoology of the nerve following axonal degeneration. University of Texas Motor axons commonly reinnervate muscle fibers by Austin, Texas 78712 growing down the old endoneurial Schwann cell tubes leading to each endplate. Some axons continue to grow beyond their endplates (Tello, 1907; Ramon y Cajal, 1928; Summary Gutmann and Young, 1944; Letinsky et al., 1976; Gorio et al., 1983), forming so-called "escaped" fibers. In some Terminal Schwann cells overlying the neuromuscular cases these escaped fibers grow to reinnervate nearby junction sprout elaborate processes upon muscle de- endplates. Muscle fibers reinnervated by an escaped fiber nervation. We show here that motor axons use these from an adjacent endplate as well as by an axon regenerat- processes as guides/substrates during regeneration; ing down the old Schwann cell tube become polyneuro- in so doing, they escape the confines of endplates and nally innervated (Rich and Lichtman, 1989). We wondered grow between endplates to generate polyneuronal in- whether the processes extended by terminal Schwann nervation. We also show that Schwann cells in the cells play a role in the generation of escaped fibers and nerve provide similar guidance. Axons extend from the polyneuronal reinnervation. Using immunocytochemistry, cut end of a nerve in association with Schwann cell we found that motor axons use processes of terminal processes and appear to navigate along them. -

Myelin Biogenesis Is Associated with Pathological Ultrastructure That Is
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Myelin biogenesis is associated with pathological ultrastructure that 2 is resolved by microglia during development 3 4 5 Minou Djannatian1,2*, Ulrich Weikert3, Shima Safaiyan1,2, Christoph Wrede4, Cassandra 6 Deichsel1,2, Georg Kislinger1,2, Torben Ruhwedel3, Douglas S. Campbell5, Tjakko van Ham6, 7 Bettina Schmid2, Jan Hegermann4, Wiebke Möbius3, Martina Schifferer2,7, Mikael Simons1,2,7* 8 9 1Institute of Neuronal Cell Biology, Technical University Munich, 80802 Munich, Germany 10 2German Center for Neurodegenerative Diseases (DZNE), 81377 Munich, Germany 11 3Max-Planck Institute of Experimental Medicine, 37075 Göttingen, Germany 12 4Institute of Functional and Applied Anatomy, Research Core Unit Electron Microscopy, 13 Hannover Medical School, 30625, Hannover, Germany 14 5Department of Neuronal Remodeling, Graduate School of Pharmaceutical Sciences, Kyoto 15 University, Sakyo-ku, Kyoto 606-8501, Japan. 16 6Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, 3015 CN, 17 the Netherlands 18 7Munich Cluster of Systems Neurology (SyNergy), 81377 Munich, Germany 19 20 *Correspondence: [email protected] or [email protected] 21 Keywords 22 Myelination, degeneration, phagocytosis, microglia, oligodendrocytes, phosphatidylserine 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
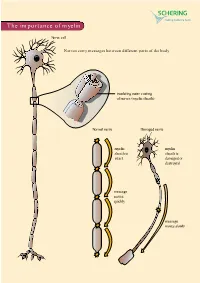
The Importance of Myelin
The importance of myelin Nerve cell Nerves carry messages between different parts of the body insulating outer coating of nerves (myelin sheath) Normal nerve Damaged nerve myelin myelin sheath is sheath is intact damaged or destroyed message moves quickly message moves slowly Nerve cells transmit impulses Nerve cells have a long, thin, flexible fibre that transmits impulses. These impulses are electrical signals that travel along the length of the nerve. Nerve fibres are long to enable impulses to travel between distant parts of the body, such as the spinal cord and leg muscles. Myelin speeds up impulses Most nerve fibres are surrounded by an insulating, fatty sheath called myelin, which acts to speed up impulses. The myelin sheath contains periodic breaks called nodes of Ranvier. By jumping from node to node, the impulse can travel much more quickly than if it had to travel along the entire length of the nerve fibre. Myelinated nerves can transmit a signal at speeds as high as 100 metres per second – as fast as a Formula One racing car. normal damaged nerve nerve Loss of myelin leads to a variety of symptoms If the myelin sheath surrounding nerve fibres is damaged or destroyed, transmission of nerve impulses is slowed or blocked. The impulse now has to flow continuously along the whole nerve fibre – a process that is much slower than jumping from node to node. Loss of myelin can also lead to ‘short-circuiting’ of nerve impulses. An area where myelin has been destroyed is called a lesion or plaque. This slowing and ‘short-circuiting’ of nerve impulses by lesions leads to a variety of symptoms related to nervous system activity. -

Glial Cells Are Involved in Itch Processing
Acta Derm Venereol 2016; 96: 723–727 REVIEW ARTICLE Glial Cells are Involved in Itch Processing Hjalte H. ANDERSEN, Lars ARENDT-NIELSEN and Parisa GAZERANI SMI®, Department of Health Science and Technology, School of Medicine, Aalborg University, Denmark Recent discoveries in itch neurophysiology include itch- worsening the skin lesions and leading to more or pro- selective neuronal pathways, the clinically relevant non- longed pruritus (5, 6). This phenomenon, known as the histaminergic pathway, and elucidation of the notable itch–scratch–itch vicious cycle, is physiologically com- similarities and differences between itch and pain. Po- plex and is likely to involve: local inflammatory medi- tential involvement of glial cells in itch processing and ators and structural changes, reward components, and the possibility of glial modulation of chronic itch have the autonomic nervous system (5, 7). In most conditions recently been identified, similarly to the established glial associated with chronic itch, very limited or ambiguous modulation of pain processing. This review outlines the evidence is found for the effectiveness of pharmaceutical similarities and differences between itch and pain, and interventions, and the evidence is often characterized by how different types of central and peripheral glial cells off-label small-scale trials or case series. Histamine is may be differentially involved in the development of ch- now widely accepted not to have a key role in evoking ronic itch akin to their more investigated role in chronic itch in most of the clinical conditions characterized by pain. Improvements are needed in the management of chronic pruritus (for an overview of itch neurobiology chronic itch, and future basic and interventional studies and mechanisms, see recent reviews in the field [8, 9]). -

Regulation of Myelin Structure and Conduction Velocity by Perinodal Astrocytes
Correction NEUROSCIENCE Correction for “Regulation of myelin structure and conduc- tion velocity by perinodal astrocytes,” by Dipankar J. Dutta, Dong Ho Woo, Philip R. Lee, Sinisa Pajevic, Olena Bukalo, William C. Huffman, Hiroaki Wake, Peter J. Basser, Shahriar SheikhBahaei, Vanja Lazarevic, Jeffrey C. Smith, and R. Douglas Fields, which was first published October 29, 2018; 10.1073/ pnas.1811013115 (Proc. Natl. Acad. Sci. U.S.A. 115,11832–11837). The authors note that the following statement should be added to the Acknowledgments: “We acknowledge Dr. Hae Ung Lee for preliminary experiments that informed the ultimate experimental approach.” Published under the PNAS license. Published online June 10, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1908361116 12574 | PNAS | June 18, 2019 | vol. 116 | no. 25 www.pnas.org Downloaded by guest on October 2, 2021 Regulation of myelin structure and conduction velocity by perinodal astrocytes Dipankar J. Duttaa,b, Dong Ho Wooa, Philip R. Leea, Sinisa Pajevicc, Olena Bukaloa, William C. Huffmana, Hiroaki Wakea, Peter J. Basserd, Shahriar SheikhBahaeie, Vanja Lazarevicf, Jeffrey C. Smithe, and R. Douglas Fieldsa,1 aSection on Nervous System Development and Plasticity, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892; bThe Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817; cMathematical and Statistical Computing Laboratory, Office of Intramural Research, Center for Information -
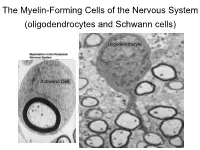
The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

Specific Labeling of Synaptic Schwann Cells Reveals Unique Cellular And
RESEARCH ARTICLE Specific labeling of synaptic schwann cells reveals unique cellular and molecular features Ryan Castro1,2,3, Thomas Taetzsch1,2, Sydney K Vaughan1,2, Kerilyn Godbe4, John Chappell4, Robert E Settlage5, Gregorio Valdez1,2,6* 1Department of Molecular Biology, Cellular Biology, and Biochemistry, Brown University, Providence, United States; 2Center for Translational Neuroscience, Robert J. and Nancy D. Carney Institute for Brain Science and Brown Institute for Translational Science, Brown University, Providence, United States; 3Neuroscience Graduate Program, Brown University, Providence, United States; 4Fralin Biomedical Research Institute at Virginia Tech Carilion, Roanoke, United States; 5Department of Advanced Research Computing, Virginia Tech, Blacksburg, United States; 6Department of Neurology, Warren Alpert Medical School of Brown University, Providence, United States Abstract Perisynaptic Schwann cells (PSCs) are specialized, non-myelinating, synaptic glia of the neuromuscular junction (NMJ), that participate in synapse development, function, maintenance, and repair. The study of PSCs has relied on an anatomy-based approach, as the identities of cell-specific PSC molecular markers have remained elusive. This limited approach has precluded our ability to isolate and genetically manipulate PSCs in a cell specific manner. We have identified neuron-glia antigen 2 (NG2) as a unique molecular marker of S100b+ PSCs in skeletal muscle. NG2 is expressed in Schwann cells already associated with the NMJ, indicating that it is a marker of differentiated PSCs. Using a newly generated transgenic mouse in which PSCs are specifically labeled, we show that PSCs have a unique molecular signature that includes genes known to play critical roles in *For correspondence: PSCs and synapses. These findings will serve as a springboard for revealing drivers of PSC [email protected] differentiation and function. -

Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease
MOG ANTIBODY DISEASE Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-Associated Encephalomyelitis WHAT IS MOG ANTIBODY-ASSOCIATED DEMYELINATION? Myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelination is an immune-mediated inflammatory process that affects the central nervous system (brain and spinal cord). MOG is a protein that exists on the outer surface of cells that create myelin (an insulating layer around nerve fibers). In a small number of patients, an initial episode of inflammation due to MOG antibodies may be the first manifestation of multiple sclerosis (MS). Most patients may only experience one episode of inflammation, but repeated episodes of central nervous system demyelination can occur in some cases. WHAT ARE THE SYMPTOMS? • Optic neuritis (inflammation of the optic nerve(s)) may be a symptom of MOG antibody- associated demyelination, which may result in painful loss of vision in one or both eyes. • Transverse myelitis (inflammation of the spinal cord) may cause a variety of symptoms that include: o Abnormal sensations. Numbness, tingling, coldness or burning in the arms and/or legs. Some are especially sensitive to the light touch of clothing or to extreme heat or cold. You may feel as if something is tightly wrapping the skin of your chest, abdomen or legs. o Weakness in your arms or legs. Some people notice that they're stumbling or dragging one foot, or heaviness in the legs. Others may develop severe weakness or even total paralysis. o Bladder and bowel problems. This may include needing to urinate more frequently, urinary incontinence, difficulty urinating and constipation. • Acute disseminated encephalomyelitis (ADEM) may cause loss of vision, weakness, numbness, and loss of balance, and altered mental status. -

Advances in Transcription Factors Related to Neuroglial Cell Reprogramming
Translational Neuroscience 2020; 11: 17–27 Review Article Kuangpin Liu, Wei Ma, Chunyan Li, Junjun Li, Xingkui Zhang, Jie Liu, Wei Liu, Zheng Wu, Chenghao Zang, Yu Liang, Jianhui Guo, Liyan Li* Advances in transcription factors related to neuroglial cell reprogramming https://doi.org/10.1515/tnsci-2020-0004 glia. Neuroglial cells are intimate partners of neurons Received August 23, 2019; accepted January 7, 2020 throughout their life cycle [2]. In embryos, neuroglial cells form a cellular framework and regulate the survival Abstract: Neuroglial cells have a high level of plasticity, and differentiation of neurons. In addition, during and many types of these cells are present in the nervous neurogenesis and early development, neuroglial cells system. Neuroglial cells provide diverse therapeutic mediate the proliferation and differentiation of neurons targets for neurological diseases and injury repair. Cell by synthesizing and secreting various growth factors and reprogramming technology provides an efficient pathway extracellular matrix components [2]. The most prominent for cell transformation during neural regeneration, while function of neuroglial cells during development is transcription factor-mediated reprogramming can facilitate formation of myelin sheaths around axons, which provide the understanding of how neuroglial cells mature into necessary signals and maintain rapid conduction for functional neurons and promote neurological function nervous system function [3]. Additionally, neuroglial recovery. cells maintain homeostasis in nerve cells and participate in synaptic plasticity and cell repair [2]. Similar to Keywords: Neuroglial cell; Reprogramming; Transcription developmental processes in other types of animal cells, factor the development of neuroglial cells is influenced by interactions between cells; cell lineage and extracellular signaling can regulate the migration, proliferation and 1 Introduction differentiation of glial cells. -

Human Nerve-On-A-Chip. Engineering 3D Functional Human Peripheral Nerve in Vitro
bioRxiv preprint doi: https://doi.org/10.1101/458463; this version posted November 8, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Human Nerve-on-a-Chip. Engineering 3D functional human peripheral nerve in vitro Anup D. Sharma1,*, Laurie McCoy1, Elizabeth Jacobs1, Hannah Willey1, Jordan Q. Behn1, Hieu Ngyuen1, Brad Bolon4, J. Lowry Curley1, Michael J. Moore1,2,3,* AxoSim Inc.1, Dept. of Biomedical Engineering2, and Brain Institute3, Tulane University, New Orleans, LA. GEMpath, Inc.,4 Longmont, CO, USA *Corresponding authors Anup Dutt Sharma Research Scientist AxoSim, Inc. 1441 Canal Street, Suite 205 New Orleans BioInnovation Center New Orleans, LA 70112 Email: [email protected] Michael J. Moore Associate Professor 526 Lindy Boggs Center Department of Biomedical Engineering Tulane University New Orleans, LA 70118 Email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/458463; this version posted November 8, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Development of ”organ-on-a-chip” systems in the space of the nervous system is lagging because of lack of availability of human neuronal & glial cells and the structural complexity of the nervous system. Furthermore, a sizeable challenge in the drug development pipeline and an often-cited reason for failure in a vast number of clinical trials conducted for neuropathological ailments is the lack of translation from animal models. A preclinical in vitro model capable of mimicking the function and structure of the human nervous system is well desired.