PREVALENCE and PATHOLOGY of RABBIT COCCIDIOSIS in NAIROBI COUNTY, KENYA. a Research Project Submitted in Partial Fulfillment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And the Winner Should Be
EDITORIAL And the winner should be… It is time that the tremendous contribution made by Carl Woese to microbiology, medicine and biology as a whole is rewarded by the Nobel committee. Among microbiologists, Carl Woese’s achievements are isolates can allow rapid disease diagnosis. As culturing is well known. His most lauded accomplishments include the not required, sequence analysis can be used to diagnose recognition of an entirely new domain of organisms, many different infectious diseases and is now common in the Archaea1, and the subsequent introduction of the clinical diagnostic laboratories. In the case of Whipple’s three-domain phylogeny2 that is now widely recognized as disease, it even led to the identification of a previously the most accurate reflection of the relatedness of all organ- unknown bacterium (Tropheryma whipplei) as the cause4. isms. We are so used to using neatly organized taxonomic Second, using 16S rRNA-based phylogeny, it is possible to trees to demonstrate the relationships between different study the human microbiota at a detailed level. In recent microorganisms that it is easy to forget that not so long ago years, we have begun to obtain a comprehensive picture the classification of bacteria was considered hopeless. In of the composition of the human-associated microbiota 1963 Roger Stanier, then one of the foremost researchers in various niches, including the gut, skin and oral cavity. in the field of bacterial classification, proclaimed that “The This has led to the insight that shifts in the gut-associated ultimate scientific goal of biological classification cannot microbiota are associated with diseases such as Crohn’s be achieved in the case of bacteria” (REF. -

Introduction to Bacteriology and Bacterial Structure/Function
INTRODUCTION TO BACTERIOLOGY AND BACTERIAL STRUCTURE/FUNCTION LEARNING OBJECTIVES To describe historical landmarks of medical microbiology To describe Koch’s Postulates To describe the characteristic structures and chemical nature of cellular constituents that distinguish eukaryotic and prokaryotic cells To describe chemical, structural, and functional components of the bacterial cytoplasmic and outer membranes, cell wall and surface appendages To name the general structures, and polymers that make up bacterial cell walls To explain the differences between gram negative and gram positive cells To describe the chemical composition, function and serological classification as H antigen of bacterial flagella and how they differ from flagella of eucaryotic cells To describe the chemical composition and function of pili To explain the unique chemical composition of bacterial spores To list medically relevant bacteria that form spores To explain the function of spores in terms of chemical and heat resistance To describe characteristics of different types of membrane transport To describe the exact cellular location and serological classification as O antigen of Lipopolysaccharide (LPS) To explain how the structure of LPS confers antigenic specificity and toxicity To describe the exact cellular location of Lipid A To explain the term endotoxin in terms of its chemical composition and location in bacterial cells INTRODUCTION TO BACTERIOLOGY 1. Two main threads in the history of bacteriology: 1) the natural history of bacteria and 2) the contagious nature of infectious diseases, were united in the latter half of the 19th century. During that period many of the bacteria that cause human disease were identified and characterized. 2. Individual bacteria were first observed microscopically by Antony van Leeuwenhoek at the end of the 17th century. -

Applications of Microscopy in Bacteriology
Microscopy Research, 2016, 4, 1-9 Published Online January 2016 in SciRes. http://www.scirp.org/journal/mr http://dx.doi.org/10.4236/mr.2016.41001 Applications of Microscopy in Bacteriology Mini Mishra1, Pratima Chauhan2* 1Centre of Environmental Studies, Department of Botany, University of Allahabad, Allahabad, India 2Department of Physics, University of Allahabad, Allahabad, India Received 28 September 2015; accepted 2 January 2016; published 5 January 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Bacteria are smallest primitive, simple, unicellular, prokaryotic and microscopic organisms. But these organisms cannot be studied with naked eyes because of their minute structure. Therefore in search for the information about the structure and composition of bacterial cells, cell biologist used light microscopes with a numerical aperture of 1.4 and using wavelength of 0.4 µm separa- tion. But there are still certain cellular structures that cannot be seen through naked eyes, and for them electron microscope is used. There are certain improved types of light microscope which can be incorporated to improve their resolving power. Hence microscopy is playing a crucial role in the field of bacteriology. Keywords AFM, SEM, TEM, Microscopy, Bacteriology 1. Introduction To get acquainted with the world of bacteria like small organisms, very effective and advanced technique is re- quired. The size of bacteria ranges between 0.5 - 5.0 micrometer in length; the smallest of them are members of mycoplasma which measures 0.3 micrometers [1]. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology. -

History of the Department of Microbiology 1868 – 2009
June 2015 HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 University of Illinois at Urbana-Champaign 1 A HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 This 141 year history of the Department of Microbiology includes an article (Chapter 1), written and published in 1959 by the Department, which covers the period 1868 to 1959. I joined the Department in 1953, and my recounting of the Department’s history includes personal observations as well as anecdotes told to me by H. O. Halvorson and others. Later I realized what a unique experience it had been to join a first-class department, and I resolved to play a role in maintaining its research stature. Ralph Wolfe 2 Department of Microbiology History of the Headship: 1950 – 1959 Halvor Halvorson 1960 – 1963 Kim Atwood 1963 – 1972 Leon Campbell 1972 – 1982 Ralph DeMoss 1982 – 1987 Samuel Kaplan 1987 – 1990 Jordan Konisky 1990 – 1991 Ralph Wolfe (Acting Head) 1991 – 1997 Charles Miller 1997 – 2002 John Cronan 2003 – 2004 Jeffrey Gardner (Acting Head) 2005 – Present John Cronan 3 Organization of the History of the Department In Chapters 2 to 6 the data are divided into Academic Decades, each containing the following sections: Section I, an overview of the decade; Section II, some events for each year of the decade; Section III, a summary of the research interests, honors received, publications, and invited off-campus lectures or seminars for each faculty member. These data have been obtained from the annual reports of the faculty submitted to the departmental secretary. 4 CHAPTER 1 1868 – 1959 During this time period the name of the Department was Department of Bacteriology (Anecdotes by Ralph Wolfe) A SHORT HISTORY OF THE DEPARTMENT OF BACTERIOLOGY H. -
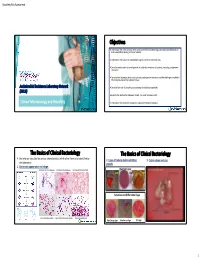
Objectives the Basics of Clinical Bacteriology the Basics of Clinical
Biosafety Risk Assessment Objectives Understand the most common tests used by the clinical bacteriology laboratory for identification and susceptibility testing of clinical isolates. Understand the classes of antimicrobial agents and their potential uses. Describe mechanisms for development of antibiotic resistance in bacteria, including carbapenem resistance. Describe the laboratory tests used to detect carbapenem resistance and the challenges involved in the interpretation of the laboratory data. Antimicrobial Resistance Laboratory Network Describe the role of biosafety in protecting the healthcare provider. (ARLN) Explain the relationship between hazard, risk, and risk assessment. Clinical Microbiology and Biosafety Understand the Antibiotic Resistance Laboratory Network initiative. The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology Bacteria are classified by various characteristics which allow them to be identified by 2. Types of culture media exhibiting 3. Colony shape and size: the laboratory: growth: 1. Gram stain appearance and shape: Gram Positive Cocci in chains (purple) Gram Positive Diplococci (purple) Gram Negative Diplococci (red/pink) Nutrient Agar BAP BAP BAP Selective and Differential Agar Gram Negative Rods(red/pink) Gram Positive Cocci in Clusters (purple) Gram Positive Rods (purple) MacConkey Agar MacConkey Agar XLD Agar 1 Biosafety Risk Assessment The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 4. Atmospheric requirements for bacterial growth: 5. Organism Identification: Spot tests – rapid biochemical tests which can be used to rule in/out various groups of organisms Catalase: Ability to breakdown H2O2 Oxidase: Presence of cytochrome oxidase CO2 – Neisseria spp., Haemophilus spp., Streptococcus pneumonia 2 Microaerophilic ( reduced O ) – Campylobacter spp. (+) Staphylococcus spp. (=) Streptococcus spp. Pseudomonas spp. E. coli (Enterobacteriaceae) Anaerobic (lack of O2) – Clostridium difficile The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 5. -

Bacteriology
SECTION 1 High Yield Microbiology 1 Bacteriology MORGAN A. PENCE Definitions Obligate/strict anaerobe: an organism that grows only in the absence of oxygen (e.g., Bacteroides fragilis). Spirochete Aerobe: an organism that lives and grows in the presence : spiral-shaped bacterium; neither gram-positive of oxygen. nor gram-negative. Aerotolerant anaerobe: an organism that shows signifi- cantly better growth in the absence of oxygen but may Gram Stain show limited growth in the presence of oxygen (e.g., • Principal stain used in bacteriology. Clostridium tertium, many Actinomyces spp.). • Distinguishes gram-positive bacteria from gram-negative Anaerobe : an organism that can live in the absence of oxy- bacteria. gen. Bacillus/bacilli: rod-shaped bacteria (e.g., gram-negative Method bacilli); not to be confused with the genus Bacillus. • A portion of a specimen or bacterial growth is applied to Coccus/cocci: spherical/round bacteria. a slide and dried. Coryneform: “club-shaped” or resembling Chinese letters; • Specimen is fixed to slide by methanol (preferred) or heat description of a Gram stain morphology consistent with (can distort morphology). Corynebacterium and related genera. • Crystal violet is added to the slide. Diphtheroid: clinical microbiology-speak for coryneform • Iodine is added and forms a complex with crystal violet gram-positive rods (Corynebacterium and related genera). that binds to the thick peptidoglycan layer of gram-posi- Gram-negative: bacteria that do not retain the purple color tive cell walls. of the crystal violet in the Gram stain due to the presence • Acetone-alcohol solution is added, which washes away of a thin peptidoglycan cell wall; gram-negative bacteria the crystal violet–iodine complexes in gram-negative appear pink due to the safranin counter stain. -

Molecular Detection of Eimeria Stiedae in an Angora Rabbit
Etlik Vet Mikrobiyol Derg, 2015; 26 (2): 41-44 Case Report / Olgu Sunumu Molecular detection of Eimeria stiedae in an Angora rabbit Armağan Erdem ÜTÜK1, Fatma Çiğdem PİŞKİN2, İbrahim BALKAYA3, Mehmet Çağrı KARAKURUM4 1 Çukurova University, Faculty of Ceyhan Veterinary Medicine, Department of Parasitology, Adana, Turkey 2 Veterinary Control Central Research Institute, Parasitology and Bee Diseases Laboratory, Ankara, Turkey 3 Ataturk University, Faculty of Veterinary Medicine, Department of Parasitology, Erzurum, Turkey 4 Mehmet Akif Ersoy University, Faculty of Veterinary Medicine, Department of Internal Medicine, Burdur, Turkey Geliş Tarihi / Received: 04.09.2015, Kabul Tarihi / Accepted: 16.10.2015 Abstract: Hepatic coccidiosis caused by Eimeria stiedae, results in severe infection, outbreaks and deaths in young rabbits. Disease is diagnosed by time consuming methods like fecal examination, oocyst sporulation and necropsy. The aim of this study was to detect E.stiedae with species specific Polymerase Chain Reaction (PCR), without waiting oocyst sporulation and necropsy. Impression smears were prepared from liver nodules and stained with giemsa. Fecal samples were collected from the intestines and examined by centrifugal flotation with salt solution to detectEimeria spp. oocysts. Then oocysts were sporulated in 2.5% (w/v) aqueous potassium dichromate (K2Cr207). Primers specific to E.stiedae were used in PCR reaction. Eimeria spp. oocysts were detected after the examination of impression smears and bowel content. E.stiedae was diagnosed after the measurement of sporulated oocyst and PCR. At the end of the study, we think that; PCR will be very valuable for the rapid and true diagnosis of hepatic coccidiosis in rabbits. Key words: Angora rabbit, Eimeria stiedae, Molecular detection, Turkey. -

Thèse Coccidiose Du Poulet
اجلمهورية اجلزائرية الدميقراطية الشعبية République Algérienne Démocratique et Populaire وزارة التعليم العايل والبحث العلمي Ministère de l’Enseignement Supérieur et de la Recherche Scientifique Université Constantine 1 جامعة قسنطينة Institut des Sciences Vétérinaires 1 معهد العلوم البيطرية Thèse Présentée en vue de l’obtention du Doctorat en Sciences Vétérinaires Option : Pathologie aviaire Coccidiose du poulet : Etude pharmacologique et immunologique Présentée Par : DJEMAI Samir Membres du jury : Gamma 횪 휸 Omicron 횶 흄 Président BENCHEIKH ELFEGOUN Professeur Université de Constantine Mohammed Chérif Examinateur BENAKHLA Ahmed Professeur Université d’El-Tarf Examinateur AISSI Meriem Professeur École Nationale Supérieure Vétérinaire- Alger Examinateur ALLOUI Nadir Professeur Université de Batna Directeur de thèse MEKROUD Abdeslam Professeur Université de Constantine Année universitaire : 2016 / 2017 قَا َل َر ُسو َل ا ه َِّلل َص هَّل ا ه َُّلل عَلَْي ِه َو َس هَّل :« اللههُ هم انْ َف ْعِِن ِب َما عَله ْمتَِِن، َوعَِل ْمِِن َما يَْن َف ُعِِن، َوا ْرُز ْقِِن ِعلْ ًما تَ ْن َف ُعِِن ِب ِه » . ]السلسةل الصحيحة-حديث رمق 3151- ا أللباين[. Le Prophète- Que la prière d'Allah et Son salut soient sur lui- disait : « Ô Allah ! Fais- moi profiter de ce que Tu m’as appris, Apprends-moi ce qui m’apporte bénéfice et Accorde-moi une science par laquelle Tu Vas me faire profiter ». [Silsila Sahiha n°3151-Cheikh Al-Albani]. Prophet Muhammad-Peace be upon him- said : « Oh Allah ! Make useful for me what you have taught me, (and) teach me knowledge that will be useful to me and grant me such knowledge that will benefit me ». [Silsila Sahiha n°3151-Cheikh Al-Albani]. -

Some Protozoa Found in the Faeces of Cattle
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Northern Iowa Proceedings of the Iowa Academy of Science Volume 34 Annual Issue Article 117 1927 Some Protozoa Found in the Faeces of Cattle Elery R. Becker Iowa State College W. W. Frye Iowa State College Copyright ©1927 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Becker, Elery R. and Frye, W. W. (1927) "Some Protozoa Found in the Faeces of Cattle," Proceedings of the Iowa Academy of Science, 34(1), 331-333. Available at: https://scholarworks.uni.edu/pias/vol34/iss1/117 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Becker and Frye: Some Protozoa Found in the Faeces of Cattle SOME PROTOZOA FOUND IN THE FAECES OF CATTLE ELERY R. BECKER AND w. w. FRYE A microscopic investigation of the faeces of cattle was under taken in search of possible cyst forms of the numerous species of ciliates which inhabit the rumen and reticulum of these animals. Of the various workers who have searched for the cysts of these protozoa only one, Liebetanz ( 1910), reports success. His tech nique, however, was so open to adverse criticism, and his figures so unconvincing, that it is doubtful if even he really saw cystic forms. -

WSC 14-15 Conf 18 Layout
Joint Pathology Center Veterinary Pathology Services WEDNESDAY SLIDE CONFERENCE 2014-2015 Conference 18 11 February 2015 Guest Moderator: Timothy K. Cooper, DVM, PhD, DACVP Penn State Milton S. Hershey Medical Center College of Medicine CASE I: A (JPC 4048645). the floor was limed before applying new shavings. Only the rabbits being raised on the Signalment: 9-week-old mixed sex commercial floor were dying. The rabbits were initially treated meat rabbits, Oryctolagus cuniculus. with amprolium for two weeks because of bloody diarrhea but the rabbits developed a bloated History: Some members of this group of 9- appearance, and so were retreated with amprolium week-old rabbits were being raised on the barn for an additional two weeks. Two days following floor because of insufficient numbers of cages. the last treatment, three rabbits were submitted for The rabbits raised on the floor were bedded on postmortem examination. shavings. The bedding was changed weekly, and 1-1. Liver, rabbit: The liver was enlarged with numerous tortuous and 1-2. Liver, rabbit: Biliary ducts are tortuous and markedly ectatic, cystic biliary duct scattered throughout. (Photo courtesy of: Animal replacing large amounts of hepatic parenchyma. (HE 6X) Health Laboratory, University of Guelph, Guelph, Ontario, Canada. http://ahl.uoguelph.ca) 1 WSC 2014-2015 large numbers of a mixture of Eimeria spp. oocysts. Histopathologic Description: Liver: There is generalized marked dilation of bile d u c t s c a u s i n g compression of the surrounding hepatic p a r e n c h y m a . Hyperplastic biliary epithelium forms papillary projections into duct lumens. -

Discovery of Bacteria by Antoni Van Leeuwenhoek D
MICROBIOLOGICAL REVIEWS, Mar. 1982, p. 121-126 Vol. 47, No. 1 0146-0749/82/010121-06$02.000/ Copyright 0 1983, American Society for Microbiology The Roles of the Sense of Taste and Clean Teeth in the Discovery of Bacteria by Antoni van Leeuwenhoek D. BARDELL Department ofBiology, Kean College ofNew Jersey, Union, New Jersey 07083 INTRODUCTION.............................................................. 121 INVESTIGATIONS ON THE SENSE OF TASTE AND THE DISCOVERY OF BACTERIA. 122 vAN LEEUWENHOEK'S PRIDE IN HIS CLEAN TEETH AND THE DEFINITIVE EVIDENCE FOR THE DISCOVERY OF BACTERA .................................... 124 CONCLUSIONS.............................................................. 125 LITERATURE CiTED ............... ............................................... 126 INTRODUCTION approach, van Leeuwenhoek observed bacteria in the course of the study. The discovery of protozoa, unicellular algae, It is true that van Leeuwenhoek's numerous unicellular fungi, and bacteria by Antoni van microscopic observations covered a broad spec- Leeuwenhoek is well recorded in standard trum of subjects, but they were not made with- books on the history of microbiology (1, 4), the out definite aim. If one reads the letters van history of biology (5, 6), and the history of Leeuwenhoek sent to the Royal Society in Lon- medicine (3). The discovery of such a variety of don, and the extant letters the Royal Society and microorganisms is the reason for books devoted individual persons sent to him, one can see that entirely to van Leeuwenhoek (2). Furthermore, he pursued investigations which he originated many microbiology and biology books, for what- because the subject interested him and also that ever purpose they were written, introductory studies were made in response to requests by textbooks or otherwise, give some attention to others to investigate a specified subject with the the discoveries.