Applications of Microscopy in Bacteriology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Bacteriology and Bacterial Structure/Function
INTRODUCTION TO BACTERIOLOGY AND BACTERIAL STRUCTURE/FUNCTION LEARNING OBJECTIVES To describe historical landmarks of medical microbiology To describe Koch’s Postulates To describe the characteristic structures and chemical nature of cellular constituents that distinguish eukaryotic and prokaryotic cells To describe chemical, structural, and functional components of the bacterial cytoplasmic and outer membranes, cell wall and surface appendages To name the general structures, and polymers that make up bacterial cell walls To explain the differences between gram negative and gram positive cells To describe the chemical composition, function and serological classification as H antigen of bacterial flagella and how they differ from flagella of eucaryotic cells To describe the chemical composition and function of pili To explain the unique chemical composition of bacterial spores To list medically relevant bacteria that form spores To explain the function of spores in terms of chemical and heat resistance To describe characteristics of different types of membrane transport To describe the exact cellular location and serological classification as O antigen of Lipopolysaccharide (LPS) To explain how the structure of LPS confers antigenic specificity and toxicity To describe the exact cellular location of Lipid A To explain the term endotoxin in terms of its chemical composition and location in bacterial cells INTRODUCTION TO BACTERIOLOGY 1. Two main threads in the history of bacteriology: 1) the natural history of bacteria and 2) the contagious nature of infectious diseases, were united in the latter half of the 19th century. During that period many of the bacteria that cause human disease were identified and characterized. 2. Individual bacteria were first observed microscopically by Antony van Leeuwenhoek at the end of the 17th century. -

Electron Microscopy and the Investigation of New Infectious Diseases
Review Electron microscopy and the investigation of new infectious diseases Alan Curry@) Objectives: To review and assess the role of electron microscopy in the investigation of new infectious diseases. Design: To design a screening strategy to maximize the likelihood of detecting new or emerging pathogens in clinical samples. Results: Electron microscopy remains a useful method of investigating some viral infections (infantile gastroenteritis, virus-induced outbreaks of gastroenteritis and skin lesions) using the negative staining technique. In addition, it remains an essential technique for the investigation of new and emerging parasitic protozoan infections in the immunocompromised patients from resin-embedded tissue biopsies. Electron microscopy can also have a useful role in the investigation of certain bacterial infections. Conclusions: Electron microscopy still has much to contribute to the investigation of new and emerging pathogens, and should be perceived as capable of producing different, but equally relevant, information compared to other investigative techniques. It is the application of a combined investigative approach using several different techniques that will further our understanding of new infectious diseases. Int J Infect Dis 2003; 7: 251-258 INTRODUCTION at individually by a skilled microscopist have con- The electron microscope was developed just before tributed to the decline of electron microscopy. Against World War II in several countries, but particularly in this background, the inevitable question must be Germany.l The dramatic increase in resolution available asked-does electron microscopy still have a useful in comparison with light microscopy promised to role to play in the investigation of emerging or new revolutionize many aspects of cell biology, virology, infectious diseases? bacteriology, mycology and protozoan parasitology. -

Second Harmonic Imaging Microscopy
170 Microsc Microanal 9(Suppl 2), 2003 DOI: 10.1017/S143192760344066X Copyright 2003 Microscopy Society of America Second Harmonic Imaging Microscopy Leslie M. Loew,* Andrew C. Millard,* Paul J. Campagnola,* William A. Mohler,* and Aaron Lewis‡ * Center for Biomedical Imaging Technology, University of Connecticut Health Center, Farmington, CT 06030-1507 USA ‡ Division of Applied Physics, Hebrew University of Jerusalem, Jerusalem 91904, Israel Second Harmonic Generation (SHG) has been developed in our laboratories as a high- resolution non-linear optical imaging microscopy (“SHIM”) for cellular membranes and intact tissues. SHG is a non-linear process that produces a frequency doubling of the intense laser field impinging on a material with a high second order susceptibility. It shares many of the advantageous features for microscopy of another more established non-linear optical technique: two-photon excited fluorescence (TPEF). Both are capable of optical sectioning to produce 3D images of thick specimens and both result in less photodamage to living tissue than confocal microscopy. SHG is complementary to TPEF in that it uses a different contrast mechanism and is most easily detected in the transmitted light optical path. It also does not arise via photon emission from molecular excited states, as do both 1- and 2-photon excited fluorescence. SHG of intrinsic highly ordered biological structures such as collagen has been known for some time but only recently has the full potential of high resolution 3D SHIM been demonstrated on live cells and tissues. For example, Figure 1 shows SHIM from microtubules in a living organism, C. elegans. The images were obtained from a transgenic nematode that expresses a ß-tubulin-green fluorescent protein fusion and Figure 1 also shows the TPEF image from this molecule for comparison. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology. -

History of the Department of Microbiology 1868 – 2009
June 2015 HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 University of Illinois at Urbana-Champaign 1 A HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 This 141 year history of the Department of Microbiology includes an article (Chapter 1), written and published in 1959 by the Department, which covers the period 1868 to 1959. I joined the Department in 1953, and my recounting of the Department’s history includes personal observations as well as anecdotes told to me by H. O. Halvorson and others. Later I realized what a unique experience it had been to join a first-class department, and I resolved to play a role in maintaining its research stature. Ralph Wolfe 2 Department of Microbiology History of the Headship: 1950 – 1959 Halvor Halvorson 1960 – 1963 Kim Atwood 1963 – 1972 Leon Campbell 1972 – 1982 Ralph DeMoss 1982 – 1987 Samuel Kaplan 1987 – 1990 Jordan Konisky 1990 – 1991 Ralph Wolfe (Acting Head) 1991 – 1997 Charles Miller 1997 – 2002 John Cronan 2003 – 2004 Jeffrey Gardner (Acting Head) 2005 – Present John Cronan 3 Organization of the History of the Department In Chapters 2 to 6 the data are divided into Academic Decades, each containing the following sections: Section I, an overview of the decade; Section II, some events for each year of the decade; Section III, a summary of the research interests, honors received, publications, and invited off-campus lectures or seminars for each faculty member. These data have been obtained from the annual reports of the faculty submitted to the departmental secretary. 4 CHAPTER 1 1868 – 1959 During this time period the name of the Department was Department of Bacteriology (Anecdotes by Ralph Wolfe) A SHORT HISTORY OF THE DEPARTMENT OF BACTERIOLOGY H. -

The Development of High Performance Liquid
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 3-23-2010 The evelopmeD nt of High Performance Liquid Chromatography Systems for the Analysis of Improvised Explosives Megan N. Bottegal Florida International University, [email protected] DOI: 10.25148/etd.FI10041603 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Recommended Citation Bottegal, Megan N., "The eD velopment of High Performance Liquid Chromatography Systems for the Analysis of Improvised Explosives" (2010). FIU Electronic Theses and Dissertations. 154. https://digitalcommons.fiu.edu/etd/154 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida THE DEVELOPMENT OF OPTIMIZED HIGH PERFORMANCE LIQUID CHROMATOGRAPHY SYSTEMS FOR THE ANALYSIS OF IMPROVISED EXPLOSIVES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Megan Nicole Bottegal 2010 To: Dean Kenneth Furton College of Arts and Sciences This dissertation, written by Megan Nicole Bottegal, and entitled The Development of Optimized High Performance Liquid Chromatography Systems for the Anlysis of Improvised Explosives, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. ____________________________________ Jose Almirall ____________________________________ John Berry ____________________________________ William Hearn ____________________________________ Fenfei Leng ____________________________________ DeEtta Mills ____________________________________ Bruce McCord, Major Professor Date of Defense: March 23, 2010 The dissertation of Megan Nicole Bottegal is approved. -
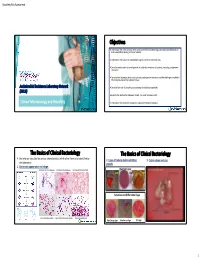
Objectives the Basics of Clinical Bacteriology the Basics of Clinical
Biosafety Risk Assessment Objectives Understand the most common tests used by the clinical bacteriology laboratory for identification and susceptibility testing of clinical isolates. Understand the classes of antimicrobial agents and their potential uses. Describe mechanisms for development of antibiotic resistance in bacteria, including carbapenem resistance. Describe the laboratory tests used to detect carbapenem resistance and the challenges involved in the interpretation of the laboratory data. Antimicrobial Resistance Laboratory Network Describe the role of biosafety in protecting the healthcare provider. (ARLN) Explain the relationship between hazard, risk, and risk assessment. Clinical Microbiology and Biosafety Understand the Antibiotic Resistance Laboratory Network initiative. The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology Bacteria are classified by various characteristics which allow them to be identified by 2. Types of culture media exhibiting 3. Colony shape and size: the laboratory: growth: 1. Gram stain appearance and shape: Gram Positive Cocci in chains (purple) Gram Positive Diplococci (purple) Gram Negative Diplococci (red/pink) Nutrient Agar BAP BAP BAP Selective and Differential Agar Gram Negative Rods(red/pink) Gram Positive Cocci in Clusters (purple) Gram Positive Rods (purple) MacConkey Agar MacConkey Agar XLD Agar 1 Biosafety Risk Assessment The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 4. Atmospheric requirements for bacterial growth: 5. Organism Identification: Spot tests – rapid biochemical tests which can be used to rule in/out various groups of organisms Catalase: Ability to breakdown H2O2 Oxidase: Presence of cytochrome oxidase CO2 – Neisseria spp., Haemophilus spp., Streptococcus pneumonia 2 Microaerophilic ( reduced O ) – Campylobacter spp. (+) Staphylococcus spp. (=) Streptococcus spp. Pseudomonas spp. E. coli (Enterobacteriaceae) Anaerobic (lack of O2) – Clostridium difficile The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 5. -

Examples of High Performance Liquid Chromatography (HPLC) Application in Marine Ecology Studies in the Northern Adriatic
Prejeto / Received: 19.6.2009 Sprejeto / Accepted: 17.12.2009 Examples of high performance liquid chromatography (HPLC) application in marine ecology studies in the northern Adriatic Vesna FLANDER-PUTRLE Marine Biology Station, National Institute of Biology, Fornače 41, SI-6330 Piran; e-mail: [email protected] Abstract. Photosynthetic pigments have proved to be useful biomarkers of the abundance, composition and physiological status of the phytoplankton biomass in the marine environment. Using HPLC pigment analysis, we determined phytoplankton community structure in three different marine environments: in the area of a fish farm, in the area of sewage outlets, and in the mucilaginous aggregates. At the reference site we observed seasonal changes with prevalence of fucoxanthin-containing phytoplankton (i.e. diatoms) during winter/spring and autumn. In the fish farm area the concentration of chlorophyll a degradation products was higher, whereas in the locally enriched environment of sewage outlets we observed only small changes in taxonomic composition and phytoplankton biomass. The impact of season is more expressed than the impact of sewage discharge. With the use of HPLC pigment analysis we determined the development of phytoplankton community in different stages of mucilage aggregates. Phytoplankton biomass was composed primarily of diatoms, and as the aggregates aged, diatoms increased in the relative biomass. Our examples have proven the usefulness and suitability of HPLC pigment analysis in marine ecology studies. Key words: pigments, HPLC, phytoplankton, Adriatic Sea, mucilage, fish farm, sewage outlets Izvleček: PRIMERI UPORABE TEKOČINSKE KROMATOGRAFIJE VISOKE LOČLJIVOSTI (HPLC) PRI ŠTUDIJAH MORSKE EKOLOGIJE SEVERNEGA JADRANA – Fotosintezna barvila so se izkazala kot dobri kazalci abundance, sestave in fiziološkega stanja fitoplanktonske biomase v morskem okolju. -

Bacteriology
SECTION 1 High Yield Microbiology 1 Bacteriology MORGAN A. PENCE Definitions Obligate/strict anaerobe: an organism that grows only in the absence of oxygen (e.g., Bacteroides fragilis). Spirochete Aerobe: an organism that lives and grows in the presence : spiral-shaped bacterium; neither gram-positive of oxygen. nor gram-negative. Aerotolerant anaerobe: an organism that shows signifi- cantly better growth in the absence of oxygen but may Gram Stain show limited growth in the presence of oxygen (e.g., • Principal stain used in bacteriology. Clostridium tertium, many Actinomyces spp.). • Distinguishes gram-positive bacteria from gram-negative Anaerobe : an organism that can live in the absence of oxy- bacteria. gen. Bacillus/bacilli: rod-shaped bacteria (e.g., gram-negative Method bacilli); not to be confused with the genus Bacillus. • A portion of a specimen or bacterial growth is applied to Coccus/cocci: spherical/round bacteria. a slide and dried. Coryneform: “club-shaped” or resembling Chinese letters; • Specimen is fixed to slide by methanol (preferred) or heat description of a Gram stain morphology consistent with (can distort morphology). Corynebacterium and related genera. • Crystal violet is added to the slide. Diphtheroid: clinical microbiology-speak for coryneform • Iodine is added and forms a complex with crystal violet gram-positive rods (Corynebacterium and related genera). that binds to the thick peptidoglycan layer of gram-posi- Gram-negative: bacteria that do not retain the purple color tive cell walls. of the crystal violet in the Gram stain due to the presence • Acetone-alcohol solution is added, which washes away of a thin peptidoglycan cell wall; gram-negative bacteria the crystal violet–iodine complexes in gram-negative appear pink due to the safranin counter stain. -

Microscopy, Optical Spectroscopy, and Macroscopic Techniques Series: Methods in Molecular Biology, Vol
C. Jones, B. Mulloy, A.H. Thomas Microscopy, Optical Spectroscopy, and Macroscopic Techniques Series: Methods in Molecular Biology, Vol. 22 This is the second of three volumes of Methods in Molecular Biology that deal with Physical Methods of Analysis. The first of these, Spectroscopic Methods and Analyses dealt with NMR spec troscopy, mass spectrometry, and metalloprotein techniques, and the third will cover X-ray crystallographic methods. As with the first volume. Microscopy, Optical Spectroscopy, and Macroscopic Techniques is intended to provide a basic understand ing for the biochemist or biologist who needs to collaborate with spe cialists in applying the techniques of modern physical chemistry to biological macromolecules. The methods treated in this book fall into four groups. Part One covers microscopy, which aims to visualize individual molecules or complexes of several molecules. Electron microscopy is the more familiar of these, while scanning tunneling microscopy is a new and rapidly developing tool. Methods for determining the shapes and sizes of molecules in solution are described in Part Two, which includes chapters on X-ray and neutron scattering, 1994, XI, 251 p. light scattering, and ult- centrifugation. Calorimetry, described in Part Three, provides the means to monitor processes involving thermodynamic changes, whether these are A product of Humana Press intramolecular, such as conformational transition, or the interactions between solutes or between a solute and its sol vent. Part Four is concerned with optical and infrared Printed book spectroscopy and describes applications ranging from the measurement of protein concentration by UV absorbance to the analysis of secondary struc ture using circular Hardcover dichroism and Fourier-transform infrared spec troscopy. -

Proximity Scanning Transmission Electron Microscopy/Spectroscopy
Proximity Scanning Transmission Electron Microscopy/Spectroscopy Ing-Shouh Hwang . Institute of Physics, Academia Sinica, Nankang, Taipei, Taiwan Email: [email protected] Abstract Here a new microscopic method is proposed to image and characterize very thin samples like few-layer materials, organic molecules, and nanostructures with nanometer or sub-nanometer resolution using electron beams of energies lower than 20 eV. The microscopic technique achieves high resolution through the proximity (or near-field) effect, as in scanning tunneling microscopy (STM), while it also allows detection of transmitted electrons for imaging and spectroscopy, as in scanning transmission electron microscopy (STEM). This proximity transmission electron microscopy (PSTEM) does not require any lens to focus the electron beam. It also allows detailed characterization of the interaction of low-energy electron with materials. PSTEM can operate in a way very similar to scanning tunneling microscopy, which provides high-resolution imaging of geometric and electronic structures of the sample surface. In addition, it allows imaging and characterization of the interior structures of the sample based on the detected transmission electron signals. PSTEM comprises a family of spectroscopies that address the transport and scattering of low-energy electrons in materials. Thus rich information can be extracted from the measurements. PSTEM offers a fundamentally new and powerful way to investigate thin materials. New analysis methods of thin materials and new physics may be uncovered by this method. Scanning tunneling microscopy (STM) [1] has been a powerful technique to study topographic and electronic properties of sample surfaces with atomic resolution. Fig. 1a illustrates a schematic of STM. A metal tip is brought to a conductive sample surface to within ~ 1 nm. -

Introduction to Confocal Laser Scanning Microscopy (LEICA)
Introduction to Confocal Laser Scanning Microscopy (LEICA) This presentation has been put together as a common effort of Urs Ziegler, Anne Greet Bittermann, Mathias Hoechli. Many pages are copied from Internet web pages or from presentations given by Leica, Zeiss and other companies. Please browse the internet to learn interactively all about optics. For questions & registration please contact www.zmb.unizh.ch . Confocal Laser Scanning Microscopy xy yz 100 µm xz 100 µm xy yz xz thick specimens at different depth 3D reconstruction Types of confocal microscopes { { { point confocal slit confocal spinning disc confocal (Nipkov) Best resolution and out-of-focus suppression as well as highest multispectral flexibility is achieved only by the classical single point confocal system ! Fundamental Set-up of Fluorescence Microscopes: confocal vs. widefield Confocal Widefield Fluorescence Fluorescence Microscopy Microscopy Photomultiplier LASER detector Detector pinhole aperture CCD Dichroic mirror Fluorescence Light Source Light source Okular pinhole aperture Fluorescence Filter Cube Objectives Sample Plane Z Focus Confocal laser scanning microscope - set up: The system is composed of a a regular florescence microscope and the confocal part, including scan head, laser optics, computer. Comparison: Widefield - Confocal Y X Higher z-resolution and reduced out-of-focus-blur make confocal pictures crisper and clearer. Only a small volume can be visualized by confocal microscopes at once. Bigger volumes need time consuming sampling and image reassembling.