Neotoma Micropus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHAPTER 1 Introduction and Historical Background
CHAPTER 1 Introduction and Historical Background Chagas disease (American trypanosomiasis) was named after the Brazilian physician Carlos Justiniano Ribeiro Chagas, who in 1909 announced to the world the discovery of this new parasitic disease in animals and humans, in the town of Lassance, State of Minas Gerais, Brazil. In 1908, Chagas observed, for the first time, flagellate forms of the parasite in the intestine of the hematophagous bug Panstrongylus megistus (initially called Conorhinus megistus), which he found residing in human dwellings in Brazil. A few months later, he studied the parasite by experimentally infecting monkeys, rodents, and dogs. At the beginning of 1909, Chagas discovered the same flagellate in the blood of a cat and in a 2-year-old girl and realized that he had discovered a new disease-causing agent, transmitted by hemi- pteran insects in the family Reduviidae, subfamily Triatominae. He named the new trypanosome Schizotrypanum cruzi, which was later renamed Trypanosoma cruzi. The enzootic condition of the new trypanosomiasis was also demonstrated by Chagas after he found a natural infection in an armadillo (Dasypus novemcinctus) and a bug (Panstrongylus geniculatus) sharing the same burrow (Chagas, 1909a, 1909b, 1912; Coura, 1997). According to the classical WHO data, it was estimated that Chagas disease affected 16–18 million people with at least 100 million at risk of contracting the infection in 21 countries throughout Latin America. There were an estimated 1 million new cases of chronic disease and some 45,000 deaths annually (WHO, 1991, 1995). Recent data indicate that these figures have been reduced drastically to less than 10 million, mainly due to the action of the various control “initiatives” throughout Latin America. -

Genetic Evidence for a Tacaribe Serocomplex Virus, Mexico
blood, samples of kidney and other solid tissues, and the Genetic Evidence skins and skeletons of the rodents were deposited into the Museum of Texas Tech University. for a Tacaribe The blood samples were tested by ELISA (10) for anti- body (immunoglobulin [Ig] G) to Whitewater Arroyo virus Serocomplex strain AV 9310135 (7). Samples of spleen and kidney from white-toothed woodrats TK133448 and TK133451, 7 oth- Virus, Mexico er white-toothed woodrats, 2 antibody-positive Nelson’s Catherine C. Inizan, Maria N. B. Cajimat, pocket mice (Chaetodipus nelsoni), and an antibody-pos- Mary Louise Milazzo, Artemio Barragán-Gomez, itive Merriam’s kangaroo rat (Dipodomys merriami) were Robert D. Bradley, and Charles F. Fulhorst tested for arenavirus by cultivation in monolayers of Vero E6 cells (11). Samples of kidney from the antibody-positive We isolated arenavirus RNA from white-toothed wood- rodents were tested for arenavirus RNA by using hemin- rats (Neotoma leucodon) captured in a region of Mexico in ested PCR assays. The fi rst-strand cDNA for the PCR was which woodrats are food for humans. Analyses of nucleotide synthesized by using SuperScript II Reverse Transcriptase and amino acid sequence data indicated that the woodrats were infected with a novel Tacaribe serocomplex virus, pro- (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA) posed name Real de Catorce virus. in conjunction with oligont 19C-cons (2). The nucleotide sequence alignments were analyzed by using MRBAYES 3.1.2 (12) in the computer software package PAUP*, ver- he Tacaribe serocomplex (family Arenaviridae, ge- sion 4.0b10 (http://paup.csit.fsu.edu). -
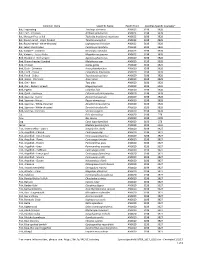
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

Ontogeny, Species Identity, and Environment Dominate Microbiome Dynamics in Wild Populations of Kissing Bugs (Triatominae) Joel J
Brown et al. Microbiome (2020) 8:146 https://doi.org/10.1186/s40168-020-00921-x RESEARCH Open Access Ontogeny, species identity, and environment dominate microbiome dynamics in wild populations of kissing bugs (Triatominae) Joel J. Brown1,2†, Sonia M. Rodríguez-Ruano1†, Anbu Poosakkannu1, Giampiero Batani1, Justin O. Schmidt3, Walter Roachell4, Jan Zima Jr1, Václav Hypša1 and Eva Nováková1,5* Abstract Background: Kissing bugs (Triatominae) are blood-feeding insects best known as the vectors of Trypanosoma cruzi, the causative agent of Chagas’ disease. Considering the high epidemiological relevance of these vectors, their biology and bacterial symbiosis remains surprisingly understudied. While previous investigations revealed generally low individual complexity but high among-individual variability of the triatomine microbiomes, any consistent microbiome determinants have not yet been identified across multiple Triatominae species. Methods: To obtain a more comprehensive view of triatomine microbiomes, we investigated the host-microbiome relationship of five Triatoma species sampled from white-throated woodrat (Neotoma albigula) nests in multiple locations across the USA. We applied optimised 16S rRNA gene metabarcoding with a novel 18S rRNA gene blocking primer to a set of 170 T. cruzi-negative individuals across all six instars. Results: Triatomine gut microbiome composition is strongly influenced by three principal factors: ontogeny, species identity, and the environment. The microbiomes are characterised by significant loss in bacterial diversity throughout ontogenetic development. First instars possess the highest bacterial diversity while adult microbiomes are routinely dominated by a single taxon. Primarily, the bacterial genus Dietzia dominates late-stage nymphs and adults of T. rubida, T. protracta, and T. lecticularia but is not present in the phylogenetically more distant T. -

Presentation Abstracts/Resúmenes De Presentaciones
1 PRESENTATION ABSTRACTS/RESÚMENES DE PRESENTACIONES WILKS AWARD COMPETITION/COMPETICIÓN PARA EL PREMIO WILKS W1 BIRD OCCUPANCY IN RELATION TO HABITAT STRUCTURE IN THE OAK SAVANNA CROSS TIMBERS OF KANSAS, USA / OCUPACION DE PAJAROS EN RELACION A ESTRUCTURAS DE HABITAT EN EL ROBLE SABANA A TRAVES DE BISQUES DE KANSAS, USA Nathan S. Holoubek and William E. Jensen Department of Biological Sciences, Emporia State University, 1200 Commercial Ave, Emporia, KS 66801 Oak savanna, once widespread across central North America, has functionally vanished from most of its range. Our objective was to quantify avian habitat associations across a gradient from open-canopy oak savanna to closed-canopy forest in the Cross Timbers region of southeastern Kansas. Using 2012 point-count data, we modeled species-specific detection (p) and occupancy (ψ) probabilities against vegetative variables using program Presence. We first established the best p predictors and then used these when modeling ψ. Of 28 species modeled, ψ for 7 was strongly associated with vegetative variables. Occupancies of Contopus virens, Polioptila caerulea, and Passerina cyanea peaked at intermediate tree densities or canopy coverages. Icterus spurius, Spiza americana, and Spizella pusilla occupancies were highest at low tree densities or canopy coverages. These species might benefit from reductions in tree density within otherwise closed-canopy forest. Moderate habitat relationships with ψ were found for three other species. Point counts will resume in 2013. Quantifying bird habitat use in oak savanna will be useful in guiding future savanna restoration for avian conservation. Roble Sabana, una vez extendido a traves de Norte America central, ha funcionalmente desaparecido en la mayoria de su extension. -

Paleoparasitology of Chagas Disease - a Review
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(Suppl. I): 9-16, 2009 9 Paleoparasitology of Chagas disease - A Review Adauto Araújo1/+, Ana Maria Jansen2, Karl Reinhard3, Luiz Fernando Ferreira1 1Escola Nacional de Saúde Pública-Fiocruz, Rua Leopoldo Bulhões 1480, 21041-210 Rio de Janeiro, RJ, Brasil 2Laboratório de Biologia de Tripanosomatídeos, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, RJ, Brasil 3School of Natural Resource Sciences, University of Nebraska-Lincoln, Lincoln, USA One hundred years since the discovery of Chagas disease associated with Trypanosoma cruzi infection, grow- ing attention has focused on understanding the evolution in parasite-human host interaction. This interest has featured studies and results from paleoparasitology, not only the description of lesions in mummified bodies, but also the recovery of genetic material from the parasite and the possibility of analyzing such material over time. The present study reviews the evidence of Chagas disease in organic remains excavated from archeological sites and discusses two findings in greater detail, both with lesions suggestive of chagasic megacolon and confirmed by molecular biology techniques. One of these sites is located in the United States, on the border between Texas and Mexico and the other in state of Minas Gerais, in the Brazilian cerrado (savannah). Dated prior to contact with Europeans, these results confirm that Chagas disease affected prehistoric human groups in other regions outside the Andean altiplanos and other transmission areas on the Pacific -

Chewing and Sucking Lice As Parasites of Iviammals and Birds
c.^,y ^r-^ 1 Ag84te DA Chewing and Sucking United States Lice as Parasites of Department of Agriculture IVIammals and Birds Agricultural Research Service Technical Bulletin Number 1849 July 1997 0 jc: United States Department of Agriculture Chewing and Sucking Agricultural Research Service Lice as Parasites of Technical Bulletin Number IVIammals and Birds 1849 July 1997 Manning A. Price and O.H. Graham U3DA, National Agrioultur«! Libmry NAL BIdg 10301 Baltimore Blvd Beltsvjlle, MD 20705-2351 Price (deceased) was professor of entomoiogy, Department of Ento- moiogy, Texas A&iVI University, College Station. Graham (retired) was research leader, USDA-ARS Screwworm Research Laboratory, Tuxtia Gutiérrez, Chiapas, Mexico. ABSTRACT Price, Manning A., and O.H. Graham. 1996. Chewing This publication reports research involving pesticides. It and Sucking Lice as Parasites of Mammals and Birds. does not recommend their use or imply that the uses U.S. Department of Agriculture, Technical Bulletin No. discussed here have been registered. All uses of pesti- 1849, 309 pp. cides must be registered by appropriate state or Federal agencies or both before they can be recommended. In all stages of their development, about 2,500 species of chewing lice are parasites of mammals or birds. While supplies last, single copies of this publication More than 500 species of blood-sucking lice attack may be obtained at no cost from Dr. O.H. Graham, only mammals. This publication emphasizes the most USDA-ARS, P.O. Box 969, Mission, TX 78572. Copies frequently seen genera and species of these lice, of this publication may be purchased from the National including geographic distribution, life history, habitats, Technical Information Service, 5285 Port Royal Road, ecology, host-parasite relationships, and economic Springfield, VA 22161. -

Detection of Bartonella and Rickettsia in Small Mammals and Their Ectoparasites in México
THERYA, 2019, Vol. 10 (2): 69-79 DOI: 10.12933/therya-19-722 ISSN 2007-3364 Detection of Bartonella and Rickettsia in small mammals and their ectoparasites in México SOKANI SÁNCHEZ-MONTES1, MARTÍN YAIR CABRERA-GARRIDO2, CÉSAR A. RÍOS-MUÑOZ1, 3, ALI ZELTZIN LIRA-OLGUIN2; ROXANA ACOSTA-GUTIÉRREZ2, MARIO MATA-GALINDO1, KEVIN HERNÁNDEZ-VILCHIS1, D. MELISSA NAVARRETE-SOTELO1, PABLO COLUNGA-SALAS1, 2, LIVIA LEÓN-PANIAGUA2 AND INGEBORG BECKER1* 1 Centro de Medicina Tropical, Unidad de Medicina Experimental, Facultad de Medicina. Universidad Nacional Autónoma de México. Dr. Balmis 148, CP. 06726, Ciudad de México. México. Email: [email protected] (SSM), [email protected] (CARM), [email protected] (MMG), [email protected] (KHV), [email protected] (MNS), colungasalas@ gmail.com (PCS), [email protected] (IB). 2 Museo de Zoología “Alfonso L. Herrera”, Departamento de Biología Evolutiva, Facultad de Ciencias, Universidad Nacional Autónoma de México. Avenida Universidad 3000, CP. 04510, Ciudad de México México. Email: [email protected]. mx (MYCG), [email protected] (AZLO), [email protected] (RAG), [email protected] (PCS), llp@ ciencias.unam.mx (LLP). 3 Laboratorio de Arqueozoología, Subdirección de Laboratorios y Apoyo Académico, Instituto Nacional de Antropología e Historia. Moneda 16, CP. 06060, Ciudad de México. México. Email: [email protected] (CARM). * Corresponding author. Fleas and sucking lice are important vectors of multiple pathogens causing major epidemics worldwide. However these insects are vec- tors of a wide range of largely understudied and unattended pathogens, especially several species of bacteria’s of the genera Bartonella and Rickettsia. For this reason the aim of the present work was to identify the presence and diversity of Bartonella and Rickettsia species in endemic murine typhus foci in Hidalgo, México. -

PROCEEDINGS of the OKLAHOMA ACADEMY of SCIENCE Volume 98 2018
PROCEEDINGS of the OKLAHOMA ACADEMY OF SCIENCE Volume 98 2018 EDITOR: Mostafa Elshahed Production Editor: Tammy Austin Business Manager: T. David Bass The Official Organ of the OKLAHOMA ACADEMY OF SCIENCE Which was established in 1909 for the purpose of stimulating scientific research; to promote fraternal relationships among those engaged in scientific work in Oklahoma; to diffuse among the citizens of the State a knowledge of the various departments of science; and to investigate and make known the material, educational, and other resources of the State. Affiliated with the American Association for the Advancement of Science. Publication Date: January 2019 ii POLICIES OF THE PROCEEDINGS The Proceedings of the Oklahoma Academy of Science contains papers on topics of interest to scientists. The goal is to publish clear communications of scientific findings and of matters of general concern for scientists in Oklahoma, and to serve as a creative outlet for other scientific contributions by scientists. ©2018 Oklahoma Academy of Science The Proceedings of the Oklahoma Academy Base and/or other appropriate repository. of Science contains reports that describe the Information necessary for retrieval of the results of original scientific investigation data from the repository will be specified in (including social science). Papers are received a reference in the paper. with the understanding that they have not been published previously or submitted for 4. Manuscripts that report research involving publication elsewhere. The papers should be human subjects or the use of materials of significant scientific quality, intelligible to a from human organs must be supported by broad scientific audience, and should represent a copy of the document authorizing the research conducted in accordance with accepted research and signed by the appropriate procedures and scientific ethics (proper subject official(s) of the institution where the work treatment and honesty). -

Updated and Revised Checklist of the Mammals of Oklahoma, 2019
1 Updated and Revised Checklist of the Mammals of Oklahoma, 2019 William Caire Biology Department, University of Central Oklahoma, Edmond, OK 73031 Lynda Samanie Loucks Biology Department, University of Central Oklahoma, Edmond, OK 73031 Michelle L. Haynie Biology Department, University of Central Oklahoma, Edmond, OK 73031 Brandi S. Coyner Sam Noble Oklahoma Museum of Natural History, Department of Mammalogy, Norman, OK 73072 Janet K. Braun Sam Noble Oklahoma Museum of Natural History, Department of Mammalogy, Norman, OK 73072 Abstract: An updated list of the mammals of Oklahoma was compiled from literature records, sight records, and museum specimens. A total of 108 native species, 4 extirpated species, and 5 introduced/exotic species are reported. jugossicularis, and Perognathus merriami), not Introduction included in the most recent checklist of Choate and Jones (1998), have been verified as occurring in the state. Choate and Jones (1998) included In a checklist of mammals of Oklahoma the domestic dog and cat as introduced/exotic (Caire et al. 1989), a total of 106 species of species which we did not. This document has mammals were listed as occurring in Oklahoma, been created in part to assist those working with including 4 extirpated and 4 introduced species. the many different and varied aspects related to In 1998, an updated checklist was published the state’s mammals. It will provide a common (Choate and Jones 1998) listing 111 species point of reference and terminology. of mammals including 4 extirpated and 7 introduced/exotic species. Since the publication Methods by Caire et al. (1989) and the updated checklist of Choate and Jones (1998), there have been To compile the updated list, we began with several changes in distributional occurrences Caire et al. -

Arthropods of Public Health Significance in California
ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA California Department of Public Health Vector Control Technician Certification Training Manual Category C ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA Category C: Arthropods A Training Manual for Vector Control Technician’s Certification Examination Administered by the California Department of Health Services Edited by Richard P. Meyer, Ph.D. and Minoo B. Madon M V C A s s o c i a t i o n of C a l i f o r n i a MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA 660 J Street, Suite 480, Sacramento, CA 95814 Date of Publication - 2002 This is a publication of the MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA For other MVCAC publications or further informaiton, contact: MVCAC 660 J Street, Suite 480 Sacramento, CA 95814 Telephone: (916) 440-0826 Fax: (916) 442-4182 E-Mail: [email protected] Web Site: http://www.mvcac.org Copyright © MVCAC 2002. All rights reserved. ii Arthropods of Public Health Significance CONTENTS PREFACE ........................................................................................................................................ v DIRECTORY OF CONTRIBUTORS.............................................................................................. vii 1 EPIDEMIOLOGY OF VECTOR-BORNE DISEASES ..................................... Bruce F. Eldridge 1 2 FUNDAMENTALS OF ENTOMOLOGY.......................................................... Richard P. Meyer 11 3 COCKROACHES ........................................................................................... -

Kissing Bugs: Not So Romantic
W 957 Kissing Bugs: Not So Romantic E. Hessock, Undergraduate Student, Animal Science Major R. T. Trout Fryxell, Associate Professor, Department of Entomology and Plant Pathology K. Vail, Professor and Extension Specialist, Department of Entomology and Plant Pathology What Are Kissing Bugs? Pest Management Tactics Kissing bugs (Triatominae), also known as cone-nosed The main goal of kissing bug management is to disrupt bugs, are commonly found in Central and South America, environments that the insects will typically inhabit. and Mexico, and less frequently seen in the southern • Focus management on areas such as your house, United States. housing for animals, or piles of debris. These insects are called “kissing bugs” because they • Fix any cracks, holes or damage to your home’s typically bite hosts around the eyes and mouths. exterior. Window screens should be free of holes to Kissing bugs are nocturnal blood feeders; thus, people prevent insect entry. experience bites while they are sleeping. Bites are • Avoid placing piles of leaves, wood or rocks within 20 usually clustered on the face and appear like other bug feet of your home to reduce possible shelter for the bites, as swollen, itchy bumps. In some cases, people insect near your home. may experience a severe allergic reaction and possibly • Use yellow lights to minimize insect attraction to anaphylaxis (a drop in blood pressure and constriction the home. of airways causing breathing difculty, nausea, vomiting, • Control or minimize wildlife hosts around a property to skin rash, and/or a weak pulse). reduce additional food sources. Kissing bugs are not specifc to one host and can feed • See UT Extension publications W 658 A Quick on a variety of animals, such as dogs, rodents, reptiles, Reference Guide to Pesticides for Pest Management livestock and birds.