2003 Baseline Inventory of Small Mammal Communities on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cross-Transmission Studies with Eimeria Arizonensis-Like Oocysts
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 6-1992 Cross-Transmission Studies with Eimeria arizonensis-like Oocysts (Apicomplexa) in New World Rodents of the Genera Baiomys, Neotoma, Onychomys, Peromyscus, and Reithrodontomys (Muridae) Steve J. Upton Kansas State University Chris T. McAllister Department of Veterans Affairs Medical Cente Dianne B. Brillhart Kansas State University Donald W. Duszynski University of New Mexico, [email protected] Constance D. Wash University of New Mexico Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Upton, Steve J.; McAllister, Chris T.; Brillhart, Dianne B.; Duszynski, Donald W.; and Wash, Constance D., "Cross-Transmission Studies with Eimeria arizonensis-like Oocysts (Apicomplexa) in New World Rodents of the Genera Baiomys, Neotoma, Onychomys, Peromyscus, and Reithrodontomys (Muridae)" (1992). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 184. https://digitalcommons.unl.edu/parasitologyfacpubs/184 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. J. Parasitol., 78(3), 1992, p. 406-413 ? American Society of Parasitologists 1992 CROSS-TRANSMISSIONSTUDIES WITH EIMERIAARIZONENSIS-LIKE OOCYSTS (APICOMPLEXA) IN NEWWORLD RODENTS OF THEGENERA BAIOMYS, NEOTOMA, ONYCHOMYS,PEROMYSCUS, AND REITHRODONTOMYS(MURIDAE) Steve J. Upton, Chris T. McAllister*,Dianne B. Brillhart,Donald W. Duszynskit, and Constance D. -

Mammalia: Rodentia) Around Amasya, Turkey
Z. ATLI ŞEKEROĞLU, H. KEFELİOĞLU, V. ŞEKEROĞLU Turk J Zool 2011; 35(4): 593-598 © TÜBİTAK Research Article doi:10.3906/zoo-0910-4 Cytogenetic characteristics of Microtus dogramacii (Mammalia: Rodentia) around Amasya, Turkey Zülal ATLI ŞEKEROĞLU1,*, Haluk KEFELİOĞLU2, Vedat ŞEKEROĞLU1 1Ordu University, Faculty of Arts and Sciences, Department of Biology, 52200 Ordu - TURKEY 2Ondokuz Mayıs University, Faculty of Arts and Sciences, Department of Biology, 55139 Kurupelit, Samsun - TURKEY Received: 02.10.2009 Abstract: Th e banding patterns of chromosomes of Microtus dogramacii, a recently described vole species endemic to Turkey, were studied. G-, C-, and Ag-NOR-banded patterns of this species are reported here for the fi rst time. In this study, 2 karyotypical forms were determined. Each form had the same diploid chromosome numbers (2n = 48), but possessed diff erent autosomal morphologies. For this reason, the samples collected from the research area were karyologically separated into 2 groups, cytotype-1 (NF = 50) and cytotype-2 (NF = 52). All chromosomes possessed centromeric/pericentromeric heterochromatin bands in both karyotypical forms. It was shown that the acrocentric chromosomes of pair 8 in cytotype-1 have been transformed into metacentric chromosomes in cytotype-2 through pericentric inversion. Variation in the number of active NORs was also observed, but the modal number of active NORs was 8. Due to the chromosomal variation found in M. dogramacii, the cytogenetic results presented in this study may represent a process of chromosomal speciation. Key words: Microtus dogramacii, karyology, pericentric inversion, Turkey Amasya (Türkiye) çevresindeki Microtus dogramacii (Mammalia: Rodentia)’nin sitogenetik özellikleri Özet: Türkiye için endemik olan yeni tanımlanmış bir tarla faresi, Microtus dogramacii’nin kromozomlarının bantlı örnekleri çalışıldı. -

Special Publications Museum of Texas Tech University Number 63 18 September 2014
Special Publications Museum of Texas Tech University Number 63 18 September 2014 List of Recent Land Mammals of Mexico, 2014 José Ramírez-Pulido, Noé González-Ruiz, Alfred L. Gardner, and Joaquín Arroyo-Cabrales.0 Front cover: Image of the cover of Nova Plantarvm, Animalivm et Mineralivm Mexicanorvm Historia, by Francisci Hernández et al. (1651), which included the first list of the mammals found in Mexico. Cover image courtesy of the John Carter Brown Library at Brown University. SPECIAL PUBLICATIONS Museum of Texas Tech University Number 63 List of Recent Land Mammals of Mexico, 2014 JOSÉ RAMÍREZ-PULIDO, NOÉ GONZÁLEZ-RUIZ, ALFRED L. GARDNER, AND JOAQUÍN ARROYO-CABRALES Layout and Design: Lisa Bradley Cover Design: Image courtesy of the John Carter Brown Library at Brown University Production Editor: Lisa Bradley Copyright 2014, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (nsrl.ttu.edu). The authors and the Museum of Texas Tech University hereby grant permission to interested parties to download or print this publication for personal or educational (not for profit) use. Re-publication of any part of this paper in other works is not permitted without prior written permission of the Museum of Texas Tech University. This book was set in Times New Roman and printed on acid-free paper that meets the guidelines for per- manence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources. Printed: 18 September 2014 Library of Congress Cataloging-in-Publication Data Special Publications of the Museum of Texas Tech University, Number 63 Series Editor: Robert J. -

Perognathus Flavescens) in Eastern Nebraska
Western North American Naturalist Volume 72 Number 4 Article 11 2-8-2013 Current status of the plains pocket mouse (Perognathus flavescens) in eastern Nebraska Keith Geluso University of Nebraska, Kearney, NE, [email protected] Greg D. Wright University of Nebraska, Kearney, NE, [email protected] Follow this and additional works at: https://scholarsarchive.byu.edu/wnan Part of the Anatomy Commons, Botany Commons, Physiology Commons, and the Zoology Commons Recommended Citation Geluso, Keith and Wright, Greg D. (2013) "Current status of the plains pocket mouse (Perognathus flavescens) in eastern Nebraska," Western North American Naturalist: Vol. 72 : No. 4 , Article 11. Available at: https://scholarsarchive.byu.edu/wnan/vol72/iss4/11 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Western North American Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Western North American Naturalist 72(4), © 2012, pp. 554–562 CURRENT STATUS OF THE PLAINS POCKET MOUSE (PEROGNATHUS FLAVESCENS) IN EASTERN NEBRASKA Keith Geluso1,3 and Greg D. Wright1,2 ABSTRACT.—Distribution of the plains pocket mouse (Perognathus flavescens) overlaps tallgrass prairies in northeastern parts of the species’ range in the central United States. Distribution and abundance of the plains pocket mouse appears negatively impacted by agricultural practices during the last century due to the scarcity of records throughout the region. In eastern Nebraska, few plains pocket mice have been captured and no published account exists in recent decades. -

Vascular Plant and Vertebrate Inventory of Chiricahua National Monument
In Cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Chiricahua National Monument Open-File Report 2008-1023 U.S. Department of the Interior U.S. Geological Survey National Park Service This page left intentionally blank. In cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Chiricahua National Monument By Brian F. Powell, Cecilia A. Schmidt, William L. Halvorson, and Pamela Anning Open-File Report 2008-1023 U.S. Geological Survey Southwest Biological Science Center Sonoran Desert Research Station University of Arizona U.S. Department of the Interior School of Natural Resources U.S. Geological Survey 125 Biological Sciences East National Park Service Tucson, Arizona 85721 U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark Myers, Director U.S. Geological Survey, Reston, Virginia: 2008 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS-the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web:http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested Citation Powell, B.F., Schmidt, C.A., Halvorson, W.L., and Anning, Pamela, 2008, Vascular plant and vertebrate inventory of Chiricahua National Monument: U.S. Geological Survey Open-File Report 2008-1023, 104 p. [http://pubs.usgs.gov/of/2008/1023/]. Cover photo: Chiricahua National Monument. Photograph by National Park Service. Note: This report supersedes Schmidt et al. (2005). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. -

Ecological Niche Evolution and Its Relation To
514 G. Shenbrot Сборник трудов Зоологического музея МГУ им. М.В. Ломоносова Archives of Zoological Museum of Lomonosov Moscow State University Том / Vol. 54 Cтр. / Pр. 514–540 ECOLOGICAL NICHE EVOLUTION AND ITS RELATION TO PHYLOGENY AND GEOGRAPHY: A CASE STUDY OF ARVICOLINE VOLES (RODENTIA: ARVICOLINI) Georgy Shenbrot Mitrani Department of Desert Ecology, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev; [email protected] Relations between ecological niches, genetic distances and geographic ranges were analyzed by pair-wise comparisons of 43 species and 38 intra- specifi c phylogenetic lineages of arvicoline voles (genera Alexandromys, Chi onomys, Lasiopodomys, Microtus). The level of niche divergence was found to be positively correlated with the level of genetic divergence and negatively correlated with the level of differences in position of geographic ranges of species and intraspecifi c forms. Frequency of different types of niche evolution (divergence, convergence, equivalence) was found to depend on genetic and geographic relations of compared forms. Among the latter with allopatric distribution, divergence was less frequent and convergence more frequent between intra-specifi c genetic lineages than between either clo sely-related or distant species. Among the forms with parapatric dis- tri bution, frequency of divergence gradually increased and frequencies of both convergence and equivalence gradually decreased from intra-specifi c genetic lineages via closely related to distant species. Among species with allopatric distribution, frequencies of niche divergence, con vergence and equivalence in closely related and distant species were si milar. The results obtained allowed suggesting that the main direction of the niche evolution was their divergence that gradually increased with ti me since population split. -

Fauna of the San Luis Valley Veryl F
New Mexico Geological Society Downloaded from: http://nmgs.nmt.edu/publications/guidebooks/22 Fauna of the San Luis Valley Veryl F. Keen, 1971, pp. 137-139 in: San Luis Basin (Colorado), James, H. L.; [ed.], New Mexico Geological Society 22nd Annual Fall Field Conference Guidebook, 340 p. This is one of many related papers that were included in the 1971 NMGS Fall Field Conference Guidebook. Annual NMGS Fall Field Conference Guidebooks Every fall since 1950, the New Mexico Geological Society (NMGS) has held an annual Fall Field Conference that explores some region of New Mexico (or surrounding states). Always well attended, these conferences provide a guidebook to participants. Besides detailed road logs, the guidebooks contain many well written, edited, and peer-reviewed geoscience papers. These books have set the national standard for geologic guidebooks and are an essential geologic reference for anyone working in or around New Mexico. Free Downloads NMGS has decided to make peer-reviewed papers from our Fall Field Conference guidebooks available for free download. Non-members will have access to guidebook papers two years after publication. Members have access to all papers. This is in keeping with our mission of promoting interest, research, and cooperation regarding geology in New Mexico. However, guidebook sales represent a significant proportion of our operating budget. Therefore, only research papers are available for download. Road logs, mini-papers, maps, stratigraphic charts, and other selected content are available only in the printed guidebooks. Copyright Information Publications of the New Mexico Geological Society, printed and electronic, are protected by the copyright laws of the United States. -

Inventory of Mammals at Walnut Canyon, Wupatki, and Sunset Crater National Monuments
National Park Service U.S. Department of the Interior Natural Resource Program Center Inventory of Mammals at Walnut Canyon, Wupatki, and Sunset Crater National Monuments Natural Resource Technical Report NPS/SCPN/NRTR–2009/278 ON THE COVER: Top: Wupatki National Monument; bottom left: bobcat (Lynx rufus); bottom right: Wupatki pocket mouse (Perogna- thus amplus cineris) at Wupatki National Monument. Photos courtesy of U.S. Geological Survey/Charles Drost. Inventory of Mammals at Walnut Canyon, Wupatki, and Sunset Crater National Monuments Natural Resource Technical Report NPS/SCPN/NRTR—2009/278 Author Charles Drost U.S. Geological Survey Southwest Biological Science Center 2255 N. Gemini Drive Flagstaff, AZ 86001 Editing and Design Jean Palumbo National Park Service, Southern Colorado Plateau Network Northern Arizona University Flagstaff, Arizona December 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Technical Report Series is used to disseminate results of scientific studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service mission. The series provides contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Genetic Evidence for a Tacaribe Serocomplex Virus, Mexico
blood, samples of kidney and other solid tissues, and the Genetic Evidence skins and skeletons of the rodents were deposited into the Museum of Texas Tech University. for a Tacaribe The blood samples were tested by ELISA (10) for anti- body (immunoglobulin [Ig] G) to Whitewater Arroyo virus Serocomplex strain AV 9310135 (7). Samples of spleen and kidney from white-toothed woodrats TK133448 and TK133451, 7 oth- Virus, Mexico er white-toothed woodrats, 2 antibody-positive Nelson’s Catherine C. Inizan, Maria N. B. Cajimat, pocket mice (Chaetodipus nelsoni), and an antibody-pos- Mary Louise Milazzo, Artemio Barragán-Gomez, itive Merriam’s kangaroo rat (Dipodomys merriami) were Robert D. Bradley, and Charles F. Fulhorst tested for arenavirus by cultivation in monolayers of Vero E6 cells (11). Samples of kidney from the antibody-positive We isolated arenavirus RNA from white-toothed wood- rodents were tested for arenavirus RNA by using hemin- rats (Neotoma leucodon) captured in a region of Mexico in ested PCR assays. The fi rst-strand cDNA for the PCR was which woodrats are food for humans. Analyses of nucleotide synthesized by using SuperScript II Reverse Transcriptase and amino acid sequence data indicated that the woodrats were infected with a novel Tacaribe serocomplex virus, pro- (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA) posed name Real de Catorce virus. in conjunction with oligont 19C-cons (2). The nucleotide sequence alignments were analyzed by using MRBAYES 3.1.2 (12) in the computer software package PAUP*, ver- he Tacaribe serocomplex (family Arenaviridae, ge- sion 4.0b10 (http://paup.csit.fsu.edu). -
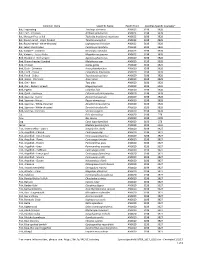
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

Mammals at Navajo National Monument
Final Report for 2003 and 2004 Mammal Inventories on Selected National Park Service Southern Colorado Plateau Network Parks: Navajo National Monument January 2005 Prepared by: Shauna Haymond, Holistic Wildlife Services NM, LLC, 112 Hampton Roads Avenue, Hampton, Virginia 23661, and Richard E. Sherwin, Department of Biology, Chemistry and Environmental Science, Christopher Newport University, 1 University Place, Newport News, Virginia 23606-2998 Submitted to: Navajo Nation Department of Fish and Wildlife, P. O. Box 1480, Window Rock, AZ 96515 ABSTRACT Holistic Wildlife Services NM was contracted by the Navajo Nation Department of Fish and Wildlife to conduct biological inventories for mammals at Navajo National Monument (NAVA) as part of the National Park Service Inventory and Monitoring Program. The goals of this study were to document at least 90% of the mammals using verifiable documentation and taxa-specific field surveys, provide distributional information, estimates of species richness, and relative abundance of mammals, and provide baseline information and make recommendations to develop future management and monitoring schemes of zoological resources. There had been no baseline mammal work conducted at NAVA prior to these surveys. A total of 26 mammal species were estimated to inhabit the park based on species-area models; however we estimated 51 species for NAVA based on known specific ranges and available museum records. Field inventories extended from 29 June to 29 September 2003, and 16 May to 5 July 2004. We used a variety of survey methods including live-trapping, mist netting and acoustic surveys for bats, track-scat surveys, and opportunistic observations. We documented a total of 41 species (Chiroptera, 12 species; Lagomorpha, 2 species; Rodentia, 18 species; Carnivora, 8 species; and Artiodactyla, 1 species). -

Studies on the Parasitic Helminths of the North Central States. I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 5-1948 Studies on the Parasitic Helminths of the North Central States. I. Helminths of Sciurida Robert L. Rausch University of Washington, [email protected] Jack Tiner University of Illinois at Urbana-Champaign Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Rausch, Robert L. and Tiner, Jack, "Studies on the Parasitic Helminths of the North Central States. I. Helminths of Sciurida" (1948). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 572. https://digitalcommons.unl.edu/parasitologyfacpubs/572 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Rausch & Tiner in American Midland Naturalist (May 1948) v. 39, no. 3. Copyright 1948, University of Notre Dame. Used by permission. Studieson theParasitic Helminths of theNorth CentralStates. 1.Helminths of Sciuridae* RobertRausch Departmentof VeterinaryScience, College of Agriculture, Universityof Wisconsin,Madison JackD. Tiner** Depa.rtmentof Zoology and Physiology,University of Illinois, Urbana It is acceptedthat a fairdegree of completenessis being achievedin our knowledgeof North Americanmammals and birds. It can be assumed that nearlyall the describedspecies of the highervertebrates are parasitizedby helminths,but many of these helminthsprobably remain undescribed,and little is known of their geographicaldistributiotn. Very little informationis availableon the incidenceof the parasites,or theireffect on the host.