WO 2017/106925 Al 29 June 2017 (29.06.2017) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Contents
TABLE OF CONTENTS ACKNOWLEDGEMENTS iii TABLE OF CONTENTS iv LIST OF TABLES vi LIST OF FIGURES viii ABSTRACT xii CHAPTER 1 INTRODUCTION 1 1.1 Overview 1 1.2 Research Objectives and Organization of the Dissertation 2 CHAPTER 2 ALKLI SILICA REACTION AND RECYCLED CONCRETE 4 AGGREGATE 2.1 Alkali Silica Reaction (ASR) 4 2.1.1 ASR essentials 4 2.1.2 ASR mechanisms 5 2.1.3 ASR test methods review 7 2.1.4 Potential mitigation methods 9 2.2 Recycled Concrete Aggregate (RCA) 11 2.2.1 Recycled aggregate preview 11 2.2.2 RCA with ASR distress 13 2.2.3 RCA alkali contribution and alkali leaching 17 CHAPTER 3 PORE SOLUTION 19 3.1 Pore solution extraction 19 3.1.1 Instrumental introduction and description 19 3.1.2 Sample preparation 24 3.1.3 Extraction method and load scheme 25 3.2 Pore Solution Chemical Analysis 27 3.2.1 Alkali evolution process in pore solution 27 3.2.2 Pore solution test schemes and methods 29 3.2.3 Test results and analysis 32 iv CHAPTER 4 RCA ASR MITIGATION USING MINERAL ADMIXTURES 35 4.1 Fly Ash Mitigation 35 4.1.1 Fly ash as a concrete admixture 35 4.1.2 Mortar bar expansion test 38 4.1.3 Expansion results and comparison 42 4.1.4 Pore solution analysis 45 4.1.5 Mitigation mechanism analysis 49 4.2 Ground Granulated Blast Furnace Slag (GGBFS) Mitigation 54 4.2.1 Slag production and use in concrete 54 4.2.2 Expansion test results and comparison 55 4.2.3 Pore solution analysis and TGA 57 4.3 Silica Fume Mitigation 59 4.3.1 Expansion results and comparison 60 4.3.2 Pore solution analysis and TGA 65 4.3.3 Particle size issue 66 4.4 -

WO 2014/138933 Al 18 September 2014 (18.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/138933 Al 18 September 2014 (18.09.2014) P O P C T (51) International Patent Classification: Jersey 08873 (US). LANGEVIN, Marie-Eve; 20 Worlds C25B 1/16 (2006.01) C25B 9/00 (2006.01) Fair Dr., Somerset, New Jersey 08873 (US). BOW 61/46 (2006.01) (74) Agent: BERESKIN & PARR LLP/S.E.N.C.R.L., S.R.L.; (21) International Application Number: 40th Floor, 40 King Street West, Scotia Plaza, Toronto, PCT/CA20 14/000264 Ontario M5H 3Y2 (CA). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 17 March 2014 (17.03.2014) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (25) Filing Language: English BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (26) Publication Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/788,292 15 March 2013 (15.03.2013) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (71) Applicant: NEMASKA LITHIUM INC. [CA/CA]; 450 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, rue de la Gare-du-Palais, ler etage, Quebec, Quebec G1K SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 3X2 (CA). -

Lithium Resources and Requirements by the Year 2000
Lithium Resources and Requirements by the Year 2000 GEOLOGICAL SURVEY PROFESSIONAL PAPER 1005 Lithium Resources and Requirements by the Year 2000 JAMES D. VINE, Editor GEOLOGICAL SURVEY PROFESSIONAL PAPER 1005 A collection of papers presented at a symposium held in Golden, Colorado, January 22-24, 1976 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director First printing 1976 Second printing 1977 Library of Congress Cataloging in Publication Data Vine, James David, 1921- Lithium resources and requirements by the year 2000. (Geological Survey Professional Paper 1005) 1. Lithium ores-United States-Congresses. 2. Lithium-Congresses. I. Vine, James David, 1921- II. Title. HI. Series: United States Geological Survey Professional Paper 1005. TN490.L5L57 553'.499 76-608206 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock Number 024-001-02887-5 CONTENTS Page 1. Introduction, by James D. Vine, U.S. Geological Survey, Denver, Colo ______________-_______-_-- — ------- —— —— ——— ---- 1 2. Battery research sponsored by the U.S. Energy Research and Development Administration, by Albert Landgrebe, Energy Research and De velopment Administration, Washington, D.C., and Paul A. Nelson, Argonne National Laboratory, Argonne, Ill-__- —— -____.—————— 2 3. Battery systems for load-leveling and electric-vehicle application, near-term and advanced technology (abstract), by N. P. Yao and W. J. Walsh, Argonne National Laboratory, Argonne, 111___.__________________________________-___-_________ — ________ 5 4. Lithium requirements for high-energy lithium-aluminum/iron-sulfide batteries for load-leveling and electric-vehicle applications, by A. -

Using Tourmaline As an Indicator of Provenance
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2016 Using Tourmaline As An Indicator Of Provenance: Development And Application Of A Statistical Approach Using Random Forests Erin Lael Walden Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Earth Sciences Commons Recommended Citation Walden, Erin Lael, "Using Tourmaline As An Indicator Of Provenance: Development And Application Of A Statistical Approach Using Random Forests" (2016). LSU Master's Theses. 4490. https://digitalcommons.lsu.edu/gradschool_theses/4490 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. USING TOURMALINE AS AN INDICATOR OF PROVENANCE: DEVELOPMENT AND APPLICATION OF A STATISTICAL APPROACH USING RANDOM FORESTS A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements of the degree of Master of Science in The Department of Geology and Geophysics by Erin Lael Walden B.S., Louisiana State University, 2008 December 2016 In memory of Wayne Douglas Walden ii ACKNOWLEDGMENTS My sincere and endless gratitude goes to Darrell Henry, who provided funding for two years and saw me through personal and academic hurdles with tireless patience and understanding. His insight and communication improved this manuscript greatly. I must also thank Barbara Dutrow for her suggestions and contributions, and Brian Marx for his expertise in statistics and help with programming. -

Zonation in Tourmaline from Granitic Pegmatites & the Occurrence of Tetrahedrally Coordinated Aluminum and Boron in Tourmaline
ZONATION IN TOURMALINE FROM GRANITIC PEGMATITES & THE OCCURRENCE OF TETRAHEDRALLY COORDINATED ALUMINUM AND BORON IN TOURMALINE by Aaron J. Lussier A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfilment of the requirements of the degree of DOCTOR OF PHILOSOPHY Department of Geological Sciences University of Manitoba Winnipeg Copyright © 2011 by Aaron J. Lussier ABSTRACT [1] Four specimens of zoned tourmaline from granitic pegmatites are characterised in detail, each having unusual compositional and/or morphologic features: (1) a crystal from Black Rapids Glacier, Alaska, showing a central pink zone of elbaite mantled by a thin rim of green liddicoatite; (2) a large (~25 cm) slab of Madagascar liddicoatite cut along (001) showing complex patterns of oscillatory zoning; and (3) a wheatsheaf and (4) a mushroom elbaite from Mogok, Myanmar, both showing extensive bifurcation of fibrous crystals originating from a central core crystal, and showing pronounced discontinuous colour zoning. Crystal chemistry and crystal structure of these samples are characterised by SREF, EMPA, and 11B and 27Al MAS NMR and Mössbauer spectroscopies. For each sample, compositional change, as a function of crystal growth, is characterised by EMPA traverses, and the total chemical variation is reduced to a series of linear substitution mechanisms. Of particular interest are substitutions accommodating the variation in [4]B: (1) TB + YAl ↔ TSi + Y(Fe, Mn)2+, where T Y T Y transition metals are present, and (2) B2 + Al ↔ Si2 + Li, where transition metals are absent. Integration of all data sets delineates constraints on melt evolution and crystal growth mechanisms. -

Winter 2007 Gems & Gemology
G EMS & G VOLUME XLIII WINTER 2007 EMOLOGY CVD Synthetic Diamonds Canary Tourmaline W Fluorescence Spectroscopy INTER Napoleon Necklace 2007 P AGES 291–408 V OLUME 43 N O. 4 THE QUARTERLY JOURNAL OF THE GEMOLOGICAL INSTITUTE OF AMERICA ® Winter 2007 VOLUME 43, NO. 4 291 LETTERS ________ FEATURE ARTICLES _____________ 294 Latest-Generation CVD-Grown Synthetic Diamonds from Apollo Diamond Inc. Wuyi Wang, Matthew S. Hall, Kyaw Soe Moe, Joshua Tower, and Thomas M. Moses Presents the gemological and spectroscopic properties of Apollo’s latest products, which show significant improvements in size, color, and clarity. 314 Yellow Mn-rich Tourmaline from the Canary Mining Area, Zambia pg. 295 Carat Points Brendan M. Laurs, William B. Simmons, George R. Rossman, Eric A. Fritz, John I. Koivula, Björn Anckar, and Alexander U. Falster Explores the vivid “canary” yellow elbaite from the Lundazi District of eastern Zambia, the most important source of this tourmaline. 332 Fluorescence Spectra of Colored Diamonds Using a Rapid, Mobile Spectrometer Sally Eaton-Magaña, Jeffrey E. Post, Peter J. Heaney, Roy A. Walters, Christopher M. Breeding, and James E. Butler Reports on the use of fluorescence spectroscopy to characterize colored diamonds from the Aurora Butterfly and other collections. NOTES AND NEW TECHNIQUES ________ 352 An Examination of the Napoleon Diamond Necklace Eloïse Gaillou and Jeffrey E. Post pg. 329 Provides a history and gemological characterization of this historic necklace. REGULAR FEATURES _____________________ 358 Lab Notes Apatite in spessartine • Atypical photoluminescence feature in a type IIa diamond • Diamond with “holiday” inclusions • Diamond with large etch channels containing iron sulfides • Black diamond with an oriented etch channel • The pareidolia of diamonds • Notable emerald carving • Gold coated onyx • Double-star sapphire • Imitation turquoise 366 Gem News International Record auction prices for diamonds • Namibian diamond mining pg. -

Molten Salts : Volume 1, Electrical Conductance, Density, and Viscosity
$ ! i v i A111D2 14bD5b NAT'L INST OF A111021 46056 * : :-• >. f. 5 i >2 > * i I v : : J, ; ; t i r t » * ? w &? * f 4 ?i ? >t»t wm W «il NBS PUBLICATIONS :• •• s r. • ? f ? ? •/? . ; f rrx: •:sn>ri r in; t 5 n * f\ p - ujuirxs) •«»': ts xrnm'ivtj: UNITED STATES DEPARTMENT OF COMMERCE C. R. Smith, Secretary NATIONAL BUREAU OF STANDARDS • A. V. Astin, Director U Molten Salts: Volume 1, Electrical Conductance, Density, and Viscosity Data G. J. Janz,* F. W. Dampier,* G. R. Lakshminarayanan,* P. K. Lorenz,* and R. P. T. Tomkins* *This report was prepared under contract at the Molten Salts Data Center Rensselaer Polytechnic Institute, Troy, N.Y. 12180 NSRDS-NBS 15 National Standard Reference Data Series- National Bureau of Standards 15 Issued October 1968 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price S3. 00 National NOV 2 9 1968 142552 Q1IOO \)^i No - /i> 6 / Library of Congress Catalog Card Number: 68-60051 Foreword The National Standard Reference Data System is a Government-wide effort to provide for the technical community of the United States effective access to the quantitative data of physical science, critically evaluated and compiled for convenience, and readily accessible through a variety of distribution channels. The System was established in 1963 by action of the President’s Office of Science and Technology and the Federal Council for Science and Technology. The responsibility to administer the System was assigned to the National Bureau of Standards and an Office of Standard Reference Data was set up at the Bureau for this purpose. -

Occurrence and Alteration of Phosphate Minerals at the Stewart
American Mineralogist, Volume 70, pages 395408, 1985 Occurrenceand alteration of phosphateminerals at the Stewart Pegmatite, Pala District,lSan Diego County, California J,c,MssE. Stncr,svr exo GonooN E. BnowN, Jn. Department of Geology St anford U niuer sity, St anford, Califurnia 94 3 0 5 Abstract Parageiibticrelationships among lithiophilite Li(Mn, Fe)2+ PO4 and its alteration products are describedfor one of the complex granitic pegmatitesin the Pala pegmatitedistrict, San Diego County, California. At the Stewart pegmatite, lithiophilite occurs in the upper- intermediate,microcline-quartz zone while spodumeneand amblygonite are found in the quartz core. The sequenceof primary mineral formation within the pegmatite reflectsan increasein the activities of both lithium speciesand volatile components(phosphorus and fluorine).Extensive alteration oflithiophilite involved oxidation, hydration, and cation leach- ing, but suprisingly little metasomatism.Secondary minerals presentinclude sicklerite,hu- reaulite, purpurite, stewartite,phosphosiderite, several incompletely identified phases,and various manganeseoxides. Secondaryphosphates formed during the initial stagesof alter- ation pseudomorphouslyreplaced lithiophilite as a result of its limited hydrothermalinterac- tion with late-stagepegmatitic fluids. Later membersof the alteration sequencerepresent supergene weathering products. The Stewart pegmatite crystallized from a highly- diflerentiatedgranitic magmaat shallowcrustal depths(about 3-5 km). The lack of extensive metasomaticreplacement, -

Ortho-Diethynylbenzene Dianion† Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Preparation of an ion with the highest calculated proton affinity: ortho-diethynylbenzene dianion† Cite this: Chem. Sci.,2016,7,6245 Berwyck L. J. Poad,ab Nicholas D. Reed,b Christopher S. Hansen,b Adam J. Trevitt,b Stephen J. Blanksby,a Emily G. Mackay,c Michael S. Sherburn,c Bun Chan‡d and Leo Radomd Owing to the increased proton affinity that results from additional negative charges, multiply-charged anions have been proposed as one route to prepare and access a range of new and powerful “superbases”. Paradoxically, while the additional electrons in polyanions increase basicity they serve to diminish the electron binding energy and thus, it had been thought, hinder experimental synthesis. We report the synthesis and isolation of the ortho-diethynylbenzene dianion (ortho-DEB2À) and present observations of this novel species undergoing gas-phase proton-abstraction reactions. Using a theoretical model based on Marcus–Hush theory, we attribute the stability of ortho-DEB2À to the Received 20th April 2016 presence of a barrier that prevents spontaneous electron detachment. The proton affinity of 1843 kJ Creative Commons Attribution 3.0 Unported Licence. Accepted 17th June 2016 molÀ1 calculated for this dianion superbase using high-level quantum chemistry calculations significantly DOI: 10.1039/c6sc01726f exceeds that of the lithium monoxide anion, the most basic system previously prepared. The ortho- www.rsc.org/chemicalscience diethynylbenzene dianion is therefore the -

Production of Lithium Peroxide and Lithium Oxide in an Alcohol Medium
PRODUCTION OF LITHIUM PEROXIDE AND LITHIUM OXIDE IN AN ALCOHOL MEDIUM by J avad Khosravi Department ofMining, Metals and Materials Engineering McGill University, Montreal, Canada March2007 A thesis submitted to the Office of Graduate and Postdoctoral Studies in fulfillment of the requirements for the degree of Doctor of Philosophy © Javad Khosravi, 2007 Libraryand Bibliothèque et 1+1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de l'édition 395 Wellington Street 395, rue Wellington Ottawa ON K1A ON4 Ottawa ON K1A ON4 Canada Canada Your file Votre référence ISBN: 978-0-494-38597-5 Our file Notre référence ISBN: 978-0-494-38597-5 NOTICE: AVIS: The author has granted a non L'auteur a accordé une licence non exclusive exclusive license allowing Library permettant à la Bibliothèque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par télécommunication ou par l'Internet, prêter, telecommunication or on the Internet, distribuer et vendre des thèses partout dans loan, distribute and sell theses le monde, à des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, électronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriété du droit d'auteur ownership and moral rights in et des droits moraux qui protège cette thèse. this thesis. Neither the thesis Ni la thèse ni des extraits substantiels de nor substantial extracts from it celle-ci ne doivent être imprimés ou autrement may be printed or otherwise reproduits sans son autorisation. -
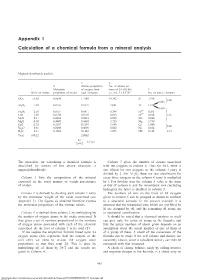
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Process for Preparing Highly Pure Lithium Carbonate
(19) TZZ ¥¥_T (11) EP 2 536 663 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C01D 15/08 (2006.01) C01B 25/10 (2006.01) 26.03.2014 Bulletin 2014/13 C01D 15/04 (2006.01) C01D 15/02 (2006.01) C01B 25/45 (2006.01) C01B 25/30 (2006.01) (2006.01) (2006.01) (21) Application number: 11707259.5 C07F 1/02 C01B 11/18 C01B 35/06 (2006.01) C07F 5/02 (2006.01) C25B 1/02 (2006.01) C25B 3/00 (2006.01) (22) Date of filing: 17.02.2011 (86) International application number: PCT/US2011/025256 (87) International publication number: WO 2011/103298 (25.08.2011 Gazette 2011/34) (54) PROCESS FOR PREPARING HIGHLY PURE LITHIUM CARBONATE VERFAHREN FÜR DIE ZUBEREITUNG VON HOCHREINEM LITHIUMCARBONAT PROCÉDÉ POUR LA PRÉPARATION DE CARBONATE DE LITHIUM DE HAUTE PURETÉ (84) Designated Contracting States: (74) Representative: Barton, Matthew Thomas AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Forresters GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Skygarden PL PT RO RS SE SI SK SM TR Erika-Mann-Strasse 11 80636 München (DE) (30) Priority: 17.02.2010 US 305213 P (56) References cited: (43) Date of publication of application: WO-A1-99/29624 DE-A1- 19 809 420 26.12.2012 Bulletin 2012/52 GB-A- 2 190 668 US-A1- 2006 115 396 (73) Proprietor: Simbol , Inc. • DATABASE WPI Week 200512 Thomson Pleasanton CA 94566 (US) Scientific, London, GB; AN 2005-109280 XP002666413, & RU 2 243 157 C2 (NOVOS CHEM (72) Inventors: CONCENTRATES STOCK CO) 27 December 2004 • HARRISON, Stephen (2004-12-27) Benicia, CA (US) • BLANCHET, Robert Palm Desert, CA (CA) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations.