Covellite Or Chalcocite??? Maine Geological Survey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract in PDF Format
PGM Associations in Copper-Rich Sulphide Ore of the Oktyabr Deposit, Talnakh Deposit Group, Russia Olga A. Yakovleva1, Sergey M. Kozyrev1 and Oleg I. Oleshkevich2 1Institute Gipronickel JS, St. Petersburg, Russia 2Mining and Metallurgical Company “Noril’sk Nickel” JS, Noril’sk, Russia e-mail: [email protected] The PGM assemblages of the Cu-rich 6%; the Ni content ranges 0.8 to 1.3%, and the ratio sulphide ores occurring in the western exocontact Cu/S = 0.1-0.2. zone of the Talnakh intrusion and the Kharaelakh Pyrrhotite-chalcopyrite ore occurs at the massive orebody have been studied. Eleven ore top of ore horizons. The ore-mineral content ranges samples, weighing 20 to 200 kg, were processed 50 to 60%, and pyrrhotite amount is <15%. The ore using gravity and flotation-gravity techniques. As a grades 1.1 to 1.3% Ni, and the ratio Cu/S = 0.35- result, the gravity concentrates were obtained from 0.7. the ores and flotation products. In the gravity Chalcopyrite ore occurs at the top and on concentrates, more than 20,000 PGM grains were the flanks of the orebodies. The concentration of found and identified, using light microscopy and sulphides ranges 50 to 60%, and pyrrhotite amount EPMA. The textural and chemical characteristics of is <1%; the Ni content ranges 1.3 to 3.4%, and the PGM were documented, as well as the PGM ratio Cu/S = 0.8-0.9. distribution in different size fractions. Also, the The ore types are distinctly distinguished balance of Pt, Pd and Au distribution in ores and by the PGE content which directly depends on the process products and the PGM mass portions in chalcopyrite quantity, but not on the total sulphide various ore types were calculated. -

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, and COBALT ARSENIDE and SULFIDE ORE FORMATION Nicholas Allin
Montana Tech Library Digital Commons @ Montana Tech Graduate Theses & Non-Theses Student Scholarship Spring 2019 EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION Nicholas Allin Follow this and additional works at: https://digitalcommons.mtech.edu/grad_rsch Part of the Geotechnical Engineering Commons EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION by Nicholas C. Allin A thesis submitted in partial fulfillment of the requirements for the degree of Masters in Geoscience: Geology Option Montana Technological University 2019 ii Abstract Experiments were conducted to determine the relative rates of reduction of aqueous sulfate and aqueous arsenite (As(OH)3,aq) using foils of copper, nickel, or cobalt as the reductant, at temperatures of 150ºC to 300ºC. At the highest temperature of 300°C, very limited sulfate reduction was observed with cobalt foil, but sulfate was reduced to sulfide by copper foil (precipitation of Cu2S (chalcocite)) and partly reduced by nickel foil (precipitation of NiS2 (vaesite) + NiSO4·xH2O). In the 300ºC arsenite reduction experiments, Cu3As (domeykite), Ni5As2, or CoAs (langisite) formed. In experiments where both sulfate and arsenite were present, some produced minerals were sulfarsenides, which contained both sulfide and arsenide, i.e. cobaltite (CoAsS). These experiments also produced large (~10 µm along longest axis) euhedral crystals of metal-sulfide that were either imbedded or grown upon a matrix of fine-grained metal-arsenides, or, in the case of cobalt, metal-sulfarsenide. Some experimental results did not show clear mineral formation, but instead demonstrated metal-arsenic alloying at the foil edges. -

Key to Rocks & Minerals Collections
STATE OF MICHIGAN MINERALS DEPARTMENT OF NATURAL RESOURCES GEOLOGICAL SURVEY DIVISION A mineral is a rock substance occurring in nature that has a definite chemical composition, crystal form, and KEY TO ROCKS & MINERALS COLLECTIONS other distinct physical properties. A few of the minerals, such as gold and silver, occur as "free" elements, but by most minerals are chemical combinations of two or Harry O. Sorensen several elements just as plants and animals are Reprinted 1968 chemical combinations. Nearly all of the 90 or more Lansing, Michigan known elements are found in the earth's crust, but only 8 are present in proportions greater than one percent. In order of abundance the 8 most important elements Contents are: INTRODUCTION............................................................... 1 Percent composition Element Symbol MINERALS........................................................................ 1 of the earth’s crust ROCKS ............................................................................. 1 Oxygen O 46.46 IGNEOUS ROCKS ........................................................ 2 Silicon Si 27.61 SEDIMENTARY ROCKS............................................... 2 Aluminum Al 8.07 METAMORPHIC ROCKS.............................................. 2 Iron Fe 5.06 IDENTIFICATION ............................................................. 2 Calcium Ca 3.64 COLOR AND STREAK.................................................. 2 Sodium Na 2.75 LUSTER......................................................................... 2 Potassium -

Leaching of Pure Chalcocite with Reject Brine and Mno2 from Manganese Nodules
metals Article Leaching of Pure Chalcocite with Reject Brine and MnO2 from Manganese Nodules David Torres 1,2,3, Emilio Trigueros 1, Pedro Robles 4 , Williams H. Leiva 5, Ricardo I. Jeldres 5 , Pedro G. Toledo 6 and Norman Toro 1,2,3,* 1 Department of Mining and Civil Engineering, Universidad Politécnica de Cartagena, 30203 Cartagena, Spain; [email protected] (D.T.); [email protected] (E.T.) 2 Faculty of Engineering and Architecture, Universidad Arturo Prat, Almirante Juan José Latorre 2901, Antofagasta 1244260, Chile 3 Departamento de Ingeniería Metalúrgica y Minas, Universidad Católica del Norte, Antofagasta 1270709, Chile 4 Escuela de Ingeniería Química, Pontificia Universidad Católica de Valparaíso, Valparaíso 2340000, Chile; [email protected] 5 Departamento de Ingeniería Química y Procesos de Minerales, Facultad de Ingeniería, Universidad de Antofagasta, Antofagasta 1270300, Chile; [email protected] (W.H.L.); [email protected] (R.I.J.) 6 Department of Chemical Engineering and Laboratory of Surface Analysis (ASIF), Universidad de Concepción, P.O. Box 160-C, Correo 3, Concepción 4030000, Chile; [email protected] * Correspondence: [email protected]; Tel.: +56-552651021 Received: 21 September 2020; Accepted: 24 October 2020; Published: 27 October 2020 Abstract: Chalcocite (Cu2S) has the fastest kinetics of dissolution of Cu in chlorinated media of all copper sulfide minerals. Chalcocite has been identified as having economic interest due to its abundance, although the water necessary for its dissolution is scarce in many regions. In this work, the replacement of fresh water by sea water or by reject brine with high chloride content from desalination plants is analyzed. -

Gomposition and Occurrence of Electrum Atthe
L37 The Canadian M inerala g i st Vol.33,pp. 137-151(1995) GOMPOSITIONAND OCCURRENCEOF ELECTRUM ATTHE MORNINGSTAR DEPOSIT, SAN BERNARDINOCOUNTY, GALIFORNIA: EVIDENCEFOR REMOBILIZATION OF GOLD AND SILVER RONALD WYNN SIIEETS*, JAMES R. CRAIG em ROBERT J. BODNAR Depanmen of Geolngical Sciences, Virginin Polytechnic h stitate and Stale (Jniversity, 4A44 Dening Hall, Blacl<sburg, Virginin 24060, U.S-A,. Arsrnacr Elecfum, acanthiteand uytenbogaardtite have been examined from six depthswithin the tabular quartzt calcite sockwork and breccia-filled veins in the fault-zone-hostedMorning Star depositof the northeasternMojave Desert, Califomia. Six distinct types of electrum have been identified on the basis of minerat association,grain moryhology and composition. Two types, (1) p1'rite-hostedand (2) quartz-hostedelectrum, occur with acanthite after argentite and base-metalsulfide minerals in unoxidized portions of the orebody; the remaining forr types, (3) goethite-hostedelectrum, (4) electnrm cores, (5) electrumrims and (6) wire electrum,are associatedwith assemblagesof supergeneminerals in its oxidizedportions. Pyrite- hosted quartz-hostedand goethite-hostedelectrum range in compositionfrom 6l ta 75 utt.7oAu and have uniform textures and no zoning. In lower portions ofthe oxidized ore zone, electrum seemsto replacegoethite and occursas small grains on surfacesof the goethite.Textural evidencefavors supergeneremobilization of Au and Ag, which were depositedas electrum on or replacinggoethite. This type of electrumis identical in appearanceand compositionto prinary electrum,In the upper portions of the oxidized zone,secondary electum occursas a gold-rich rim on a core of elechum and as wire-like grains,both with acanthiteand uytenbogaardtite.Such secondaryelectrum contains from 78 to 93 wt./o Au. Textural relations and asso- ciated minerals suggestthat the primary electrum was hydrothermally depositedand partially remobilized by supergene processes. -
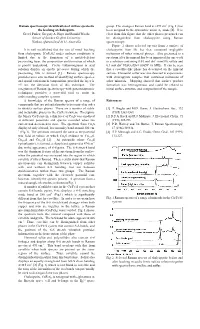
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
THE AMERICAN MINERALOGIST, VOL. 55, MAY_JUNE, 1970 AN OCCURRENCE OF THE ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.s"",AUCANQUILCHA, CHILE AraN H. Cr-etr<, Deportmentof GeologicalSciences, Queen's U niaersity, Kingston, Ontario. AssrnA.cr A mineral with composition near to CusFeSo has been found associated with nzrtive sulfur and covellite in the volcanic sulfur deposit at Aucanquilcha. Microprobe, optical and X-ray powder diffraction data match closell'a previously reported occurrence at Nu- kundamu, Fiji. Comparison with equilibrium synthesis by Kullerud and others indir:ates formation in the temperature range 434-482"C. INrnonucrroN Electron probe microanalysis(L6vy, 1967; Sillitoe and Clark, lt69) of naturally-occurring,supergene idaite has cast considerabledoub1. on the equation (Frenzel, 1959) of this not uncommon sulfi.de with the phaseof generalformula Cur r,Fe,Se.s,1 (Yund, 1963), which has h,een synthesizedby Merwin and Lombard (1937), Roseboomand Kullerud (1958),and Yund and Kullerud (1966).Idaite has been shown to have the compositionCurFeSa, or Cu3FeS4-,1orrd there is as yet no evidence of solid solution in nature between this and more copper-rich comF,osi- tions.The well-establishedX-ray powder data"(a:3.772 A; c:11.1U A; Yund, 1963) for hexagonal Cus.s,Fe"Se.r, are only with difficulty recon- ciled with thoseof natural idaite (Frenzel,1959, 1963), and L6vy (I\167) has suggestedthat Frenzel'soriginal powder data may be adequately fitted to a stannite-type,tetragonal cell. A differencein crystal struclure between thesephases is supported by the dissimilar reflectivity disper- sion profi.lesof natural idaite (L6vy, 1967; Sillitoe and Clark, 1969) and the synthetic "Cu5FeS6"of Merwin and l-ombard (1937; inLlvy, 1967). -

Effects of Copper on Fish and Aquatic Resources
Effects of Copper on Fish and Aquatic Resources Prepared for By Dr. Carol Ann Woody & Sarah Louise O’Neal Fisheries Research and Consulting Anchorage, Alaska June 2012 Effects of Copper on Fish and Aquatic Resources Introduction The Nushagak and Kvichak river watersheds in Bristol Bay Alaska (Figure 1) together produced over 650 million sockeye salmon during 1956-2011, about 40% of Bristol Bay production (ADFG 2012). Proposed mining of copper–sulfide ore in these watersheds will expose rocks with elevated metal concentrations including copper (Cu) (Figure 1; Cox 1996, NDM 2005a, Ghaffari et al. 2011). Because mining can increase metal concentrations in water by several orders of magnitude compared to uncontaminated sites (ATSDR 1990, USEPA 2000, Younger 2002), and because Cu can be highly toxic to aquatic life (Eisler 2000), this review focuses on risks to aquatic life from potential increased Cu inputs from proposed development. Figure 1. Map showing current mining claims (red) in Nushagak and Kvichak river watersheds as of 2011. Proven low- grade copper sulfide deposits are located in large lease block along Iliamna Lake. Documented salmon streams are outlined in dark blue. Note many regional streams have never been surveyed for salmon presence or absence. Sources: fish data from: www.adfg.alaska.gov/sf/SARR/AWC/index.cfm?ADFG =main.home mine data from Alaska Department of Natural Resources - http://www.asgdc.state.ak.us/ Core samples collected from Cu prospects near Iliamna Lake (Figure 1) show high potential for acid generation due to iron sulfides in the rock (NDM 2005a). When sulfides are exposed to oxygen and water sulfuric acid forms, which can dissolve metals in rock. -

Oxidation of Sulfide Minerals. V. Galena, Sphalerite and Chalcogite
Canadian Mineralogist Vol. 18,pp. 365-372(1980) OXIDATIONOF SULFIDEMINERALS. V. GALENA,SPHALERITE AND CHALCOGITE H.F. STEGBR eup L.E. DESJARDINS Mineral SciencesLaboratories, Canada Centre lor Mineral and Energy Technology, Department ol Energy, Mines and Resources,Ottawa, Ontaio KIA OGI AssrRecr long-term stability of sulfide-bearing ores and concentrates.Part of this study was concerned Samples of galena, sphalerite and cbalcocite were with the nature of the products and kinetics of oxidized at 52oC and, 68Vo of relative humidity the oxidation of the commonly encountered periods to five weeks, and the prqd- in air for up sulfide minerals. The oxidation of pyrite, chal- for metal and sulfur-bearing ucts were analyzed pyrrhotite at 52oC and, 687o of. species. Galena is. oxidized to PbSOa, sphalerite copyrite and (RH) has already been in- to ZnSO. * FezO, if iron-bearing, and chalcocite relative humidity to CuO and CuS. The oxidation of galena and vestigated (Steger & Desjardins 1978). This re- sphalerite proceeds according to a linear rate potr summarizes the results of the study 9f tle law: that of chalcocite leads to the formation of a bxidation of galena, sphalerite and chalcocite coherent product layer impenetrable to Oz and HrO under the same conditions. vapor. The air oxidation of galena at relatively low without Keywords: air oxidation, oxidation products, sul- temperatures has been investigated fide minerals, galena, sphalerite, chalcocite. reaching a consensuson the nature of the oxida- tion product. Hagihara (1952), using a re- Sourvrlrnp flection electron-diffraction technique, and Kirkwood & Nutting (1965), using a trans- Nous avons 6tudi6 I'oxydation dans I'air d'6chan- mission electron-diffraction technique, found tillons de galdne, de sphal6rite et de chalcocite i this product to be PbSOo,whereas Leia et al. -

Copper Deposits in Sedimentary and Volcanogenic Rocks
Copper Deposits in Sedimentary and Volcanogenic Rocks GEOLOGICAL SURVEY PROFESSIONAL PAPER 907-C COVER PHOTOGRAPHS 1 . Asbestos ore 8. Aluminum ore, bauxite, Georgia 1 2 3 4 2. Lead ore. Balmat mine, N . Y. 9. Native copper ore, Keweenawan 5 6 3. Chromite-chromium ore, Washington Peninsula, Mich. 4. Zinc ore, Friedensville, Pa. 10. Porphyry molybdenum ore, Colorado 7 8 5. Banded iron-formation, Palmer, 11. Zinc ore, Edwards, N.Y. Mich. 12. Manganese nodules, ocean floor 9 10 6. Ribbon asbestos ore, Quebec, Canada 13. Botryoidal fluorite ore, 11 12 13 14 7. Manganese ore, banded Poncha Springs, Colo. rhodochrosite 14. Tungsten ore, North Carolina Copper Deposits in Sedimentary and Volcanogenic Rocks By ELIZABETH B. TOURTELOT and JAMES D. VINE GEOLOGY AND RESOURCES OF COPPER DEPOSITS GEOLOGICAL SURVEY PROFESSIONAL PAPER 907-C A geologic appraisal of low-temperature copper deposits formed by syngenetic, diagenetic, and epigenetic processes UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director First printing 1976 Second printing 1976 Library of Congress Cataloging in Publication Data Tourtelot, Elizabeth B. Copper deposits in sedimentary and volcanogenic rocks. (Geology and resources of copper) (Geological Survey Professional Paper 907-C) Bibliography: p. Supt. of Docs. no.: I 19.16:907-C 1. Copper ores. 2. Rocks, Sedimentary. 3. Rocks, Igneous. I. Vine, James David, 1921- joint author. II. Title. III. Series. IV. Series: United States Geological Survey Professional Paper 907-C. TN440.T68 553'.43 76-608039 For sale by the Superintendent of Documents, U.S. -

Porphyry Deposits
PORPHYRY DEPOSITS W.D. SINCLAIR Geological Survey of Canada, 601 Booth St., Ottawa, Ontario, K1A 0E8 E-mail: [email protected] Definition Au (±Ag, Cu, Mo) Mo (±W, Sn) Porphyry deposits are large, low- to medium-grade W-Mo (±Bi, Sn) deposits in which primary (hypogene) ore minerals are dom- Sn (±W, Mo, Ag, Bi, Cu, Zn, In) inantly structurally controlled and which are spatially and Sn-Ag (±W, Cu, Zn, Mo, Bi) genetically related to felsic to intermediate porphyritic intru- Ag (±Au, Zn, Pb) sions (Kirkham, 1972). The large size and structural control (e.g., veins, vein sets, stockworks, fractures, 'crackled zones' For deposits with currently subeconomic grades and and breccia pipes) serve to distinguish porphyry deposits tonnages, subtypes are based on probable coproduct and from a variety of deposits that may be peripherally associat- byproduct metals, assuming that the deposits were econom- ed, including skarns, high-temperature mantos, breccia ic. pipes, peripheral mesothermal veins, and epithermal pre- Geographical Distribution cious-metal deposits. Secondary minerals may be developed in supergene-enriched zones in porphyry Cu deposits by weathering of primary sulphides. Such zones typically have Porphyry deposits occur throughout the world in a series significantly higher Cu grades, thereby enhancing the poten- of extensive, relatively narrow, linear metallogenic tial for economic exploitation. provinces (Fig. 1). They are predominantly associated with The following subtypes of porphyry deposits are Mesozoic to Cenozoic orogenic belts in western North and defined according to the metals that are essential to the eco- South America and around the western margin of the Pacific nomics of the deposit (metals that are byproducts or poten- Basin, particularly within the South East Asian Archipelago.