Chromatin Restriction by the Nucleosome Remodeler Mi-2Β and Functional Interplay with Lineage-Specific Transcription Regulators Control B-Cell Differentiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PLXNB1 (Plexin
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Gene Section Mini Review PLXNB1 (plexin B1) José Javier Gómez-Román, Montserrat Nicolas Martínez, Servando Lazuén Fernández, José Fernando Val-Bernal Department of Anatomical Pathology, Marques de Valdecilla University Hospital, Medical Faculty, University of Cantabria, Santander, Spain (JJGR, MN, SL, JFVB) Published in Atlas Database: March 2009 Online updated version: http://AtlasGeneticsOncology.org/Genes/PLXNB1ID43413ch3p21.html DOI: 10.4267/2042/44702 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2010 Atlas of Genetics and Cytogenetics in Oncology and Haematology Identity Pseudogene No. Other names: KIAA0407; MGC149167; OTTHUMP00000164806; PLEXIN-B1; PLXN5; SEP Protein HGNC (Hugo): PLXNB1 Location: 3p21.31 Description Local order: The Plexin B1 gene is located between 2135 Amino acids (AA). Plexins are receptors for axon FBXW12 and CCDC51 genes. molecular guidance molecules semaphorins. Plexin signalling is important in pathfinding and patterning of both neurons and developing blood vessels. Plexin-B1 is a surface cell receptor. When it binds to its ligand SEMA4D it activates several pathways by binding of cytoplasmic ligands, like RHOA activation and subsequent changes of the actin cytoskeleton, axon guidance, invasive growth and cell migration. It monomers and heterodimers with PLXNB2 after proteolytic processing. Binds RAC1 that has been activated by GTP binding. It binds PLXNA1 and by similarity ARHGEF11, Note ARHGEF12, ERBB2, MET, MST1R, RND1, NRP1 Size: 26,200 bases. and NRP2. Orientation: minus strand. This family features the C-terminal regions of various plexins. The cytoplasmic region, which has been called DNA/RNA a SEX domain in some members of this family is involved in downstream signalling pathways, by Description interaction with proteins such as Rac1, RhoD, Rnd1 and other plexins. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -
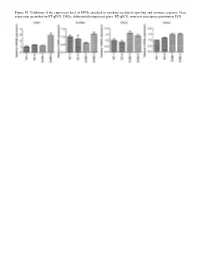
Figure S1. Validation of the Expression Level of Degs Enriched in Cytokine‑Mediated Signaling and Immune Response
Figure S1. Validation of the expression level of DEGs enriched in cytokine‑mediated signaling and immune response. Gene expression quantified by RT‑qPCR. DEGs, differentially expressed genes; RT‑qPCR, reverse‑transcription quantitative PCR. Figure S2. Validation of STAU1‑regulated AS events. IGV‑Sashimi plot revealed (A‑C) three A5SS AS events in three different genes. Reads distribution of each AS event was plotted in the left panel with the transcripts of each gene shown below. The sche‑ matic diagrams depict the structures of two AS events, AS1 (purple line) and AS2 (green line). The exon sequences are denoted by black boxes, the intron sequences by a horizontal line (right panel). RNA‑seq quantification and RT‑qPCR validation of ASEs are presented in the panels on the right. STAU1, double‑stranded RNA‑binding protein Staufen homolog 1; AS, alternative splicing; A5SS, alternative 5'splice site; RNA‑seq, RNA sequencing; RT‑qPCR, reverse‑transcription quantitative PCR. Error bars represent mean ± SEM. *P<0.05. Table SI. Primers used in gene validation experiments. IFIT2‑F CAGCCTACGGCAACTAAA IFIT2‑R GAGCCTTCTCAAAGCACA IFIT3‑F ACACCAAACAATGGCTAC IFIT3‑R TGGACAAACCCTCTAAAC OASL‑F AATGGTGACCGTGATGGG OASL‑R ACCTGAGGATGGAGCAGAG IFI27‑F TTCACTGCGGCGGGAATC IFI27‑R TGGCTGCTATGGAGGACGAG S1PR4‑F TGCTGAAGACGGTGCTGATG S1PR4‑R TGCGGAAGGAGTAGATGATGG CCL5‑F ACGACTGCTGGGTTGGAG CCL5‑R ACCCTGCTGCTTTGCCTA CCL2‑F CTAACCCAGAAACATCCAAT CCL2‑R GCTATGAGCAGCAGGCAC CD44‑F TGGAGGACAGAAAGCCAAGT CD44‑R TTCGCAATGAAACAATCAGTAG PLEKHG2‑M/As‑F CCAAAAGTAAGCCTGTCC PLEKHG2‑M‑R -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Suppl Fig 2 PB2 EUCOMM V3
Maier et al. Supplementary material Semaphorin 4C and 4G are Plexin-B2 ligands required in cerebellar development 2 Supplementary tables 4 Supplementary figures 1 Supplementary Table 1. Exencephaly and postnatal survival of Sema4C mutants (on C57BL/6 background) Genotype Expected frequency Observed frequency Exencephaly Embryonic stages (n=47)* Wild type 25% 25% (12/47) 0% Sema4c+/- 50% 45% (21/47) 0% Sema4c-/- 25% 30% (14/47) 36% (5/14) Postnatal animals (n=417)* Wild type 25% 27% (113/417) Sema4c+/- 50% 61% (253/417) Sema4c-/- 25% 12% (51/417) * Offspring from Sema4c+/- x +/- matings. Embryonic stages scored at E15-E18, postnatal animals scored at P21. 2 Supplementary Table 2. Cerebellar phenotypes of Sema4C and Sema4G mutants (on CD-1 outbred background) Ectopic Fusion of Gap in Normal Gap in IGL granule cells lobules IGL of cerebellum of lobule II in molecular VIII/IX* lobule X Genotype n layer weak strong Wild type 17 100% 0% 0% 0% 0% 0% Sema4c+/- 11 70% 10% 0% 10% 0% 10% Sema4c-/- 10 20% 70% 0% 40% 0% 40% Sema4c+/-; Sema4g+/- 16 55% 18% 0% 18% 0% 18% Sema4c+/-; Sema4g-/- 13 40% 20% 0% 20% 0% 40% Sema4c-/-; Sema4g+/- 10 20% 30% 0% 50% 0% 60% Sema4c-/-; Sema4g-/- 11 0% 55% 0% 55% 55% 82% *Fusions of lobules VIII and IX were scored as “weak” when ectopic granule cells formed a band of cells at the fusion line, and as “strong” when a continuous bridge of granule cells connected the IGL of the two lobules. 3 Supplementary figure legends Maier et al. Suppl. Fig. -

The Human Gene Connectome As a Map of Short Cuts for Morbid Allele Discovery
The human gene connectome as a map of short cuts for morbid allele discovery Yuval Itana,1, Shen-Ying Zhanga,b, Guillaume Vogta,b, Avinash Abhyankara, Melina Hermana, Patrick Nitschkec, Dror Friedd, Lluis Quintana-Murcie, Laurent Abela,b, and Jean-Laurent Casanovaa,b,f aSt. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY 10065; bLaboratory of Human Genetics of Infectious Diseases, Necker Branch, Paris Descartes University, Institut National de la Santé et de la Recherche Médicale U980, Necker Medical School, 75015 Paris, France; cPlateforme Bioinformatique, Université Paris Descartes, 75116 Paris, France; dDepartment of Computer Science, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; eUnit of Human Evolutionary Genetics, Centre National de la Recherche Scientifique, Unité de Recherche Associée 3012, Institut Pasteur, F-75015 Paris, France; and fPediatric Immunology-Hematology Unit, Necker Hospital for Sick Children, 75015 Paris, France Edited* by Bruce Beutler, University of Texas Southwestern Medical Center, Dallas, TX, and approved February 15, 2013 (received for review October 19, 2012) High-throughput genomic data reveal thousands of gene variants to detect a single mutated gene, with the other polymorphic genes per patient, and it is often difficult to determine which of these being of less interest. This goes some way to explaining why, variants underlies disease in a given individual. However, at the despite the abundance of NGS data, the discovery of disease- population level, there may be some degree of phenotypic homo- causing alleles from such data remains somewhat limited. geneity, with alterations of specific physiological pathways under- We developed the human gene connectome (HGC) to over- come this problem. -

A Robust Estimation of Exon Expression To
Risueño et al. BMC Genomics 2014, 15:879 http://www.biomedcentral.com/1471-2164/15/879 METHODOLOGY ARTICLE Open Access A robust estimation of exon expression to identify alternative spliced genes applied to human tissues and cancer samples Alberto Risueño1,5, Beatriz Roson-Burgo1,2, Anna Dolnik3, Jesus M Hernandez-Rivas2,4, Lars Bullinger3 and Javier De Las Rivas1,2* Abstract Background: Accurate analysis of whole-gene expression and individual-exon expression is essential to characterize different transcript isoforms and identify alternative splicing events in human genes. One of the omic technologies widely used in many studies on human samples are the exon-specific expression microarray platforms. Results: Since there are not many validated comparative analyses to identify specific splicing events using data derived from these types of platforms, we have developed an algorithm (called ESLiM) to detect significant changes in exon use, and applied it to a reference dataset of 270 human genes that show alternative expression in different tissues. We compared the results with three other methodological approaches and provided the R source code to be applied elsewhere. The genes positively detected by these analyses also provide a verified subset of human genes that present tissue-regulated isoforms. Furthermore, we performed a validation analysis on human patient samples comparing two different subtypes of acute myeloid leukemia (AML) and we experimentally validated the splicing in several selected genes that showed exons with highly significant signal change. Conclusions: The comparative analyses with other methods using a fair set of human genes that show alternative splicing and the validation on clinical samples demonstrate that the proposed novel algorithm is a reliable tool for detecting differential splicing in exon-level expression data. -

Downloaded, Each with Over 20 Samples for AD-Specific Pathways, Biological Processes, and Driver Each Specific Brain Region in Each Condition
Xiang et al. BMC Medical Genomics 2018, 11(Suppl 6):115 https://doi.org/10.1186/s12920-018-0431-1 RESEARCH Open Access Condition-specific gene co-expression network mining identifies key pathways and regulators in the brain tissue of Alzheimer’s disease patients Shunian Xiang1,2, Zhi Huang4, Wang Tianfu1, Zhi Han3, Christina Y. Yu3,5, Dong Ni1*, Kun Huang3* and Jie Zhang2* From 29th International Conference on Genome Informatics Yunnan, China. 3-5 December 2018 Abstract Background: Gene co-expression network (GCN) mining is a systematic approach to efficiently identify novel disease pathways, predict novel gene functions and search for potential disease biomarkers. However, few studies have systematically identified GCNs in multiple brain transcriptomic data of Alzheimer’s disease (AD) patients and looked for their specific functions. Methods: In this study, we first mined GCN modules from AD and normal brain samples in multiple datasets respectively; then identified gene modules that are specific to AD or normal samples; lastly, condition-specific modules with similar functional enrichments were merged and enriched differentially expressed upstream transcription factors were further examined for the AD/normal-specific modules. Results: We obtained 30 AD-specific modules which showed gain of correlation in AD samples and 31 normal- specific modules with loss of correlation in AD samples compared to normal ones, using the network mining tool lmQCM. Functional and pathway enrichment analysis not only confirmed known gene functional categories related to AD, but also identified novel regulatory factors and pathways. Remarkably, pathway analysis suggested that a variety of viral, bacteria, and parasitic infection pathways are activated in AD samples. -

Plexin-B1 Antagonists and Uses Thereof Plexin-B1 Antagonisten Und Verwendungen Davon Antagonistes De Plexine B1 Et Utilisations De Ceux-Ci
(19) TZZ ¥¥ _T (11) EP 2 673 362 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/113 (2010.01) A61K 31/713 (2006.01) 07.06.2017 Bulletin 2017/23 A61P 35/04 (2006.01) A61K 39/395 (2006.01) C07K 16/30 (2006.01) (21) Application number: 12706220.6 (86) International application number: (22) Date of filing: 09.02.2012 PCT/EP2012/052238 (87) International publication number: WO 2012/107531 (16.08.2012 Gazette 2012/33) (54) PLEXIN-B1 ANTAGONISTS AND USES THEREOF PLEXIN-B1 ANTAGONISTEN UND VERWENDUNGEN DAVON ANTAGONISTES DE PLEXINE B1 ET UTILISATIONS DE CEUX-CI (84) Designated Contracting States: • DEAGLIO SILVIA ET AL: "CD38 and CD100 lead AL AT BE BG CH CY CZ DE DK EE ES FI FR GB a network of surface receptors relaying positive GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO signals for B-CLL growth and survival", BLOOD, PL PT RO RS SE SI SK SM TR AMERICAN SOCIETY OFHEMATOLOGY, US, vol. 105, no. 8, 1 April 2005 (2005-04-01) , pages (30) Priority: 09.02.2011 EP 11153784 3042-3050, XP002405817, ISSN: 0006-4971, DOI: 10.1182/BLOOD-2004-10-3873 (43) Date of publication of application: • SWIERCZ JAKUB M ET AL: "Semaphorin 4D 18.12.2013 Bulletin 2013/51 signaling requires the recruitment of phospholipase C gamma into the plexin-B1 (73) Proprietors: receptor complex.", MOLECULAR AND • Ruprecht-Karls-Universität Heidelberg CELLULAR BIOLOGY, vol. 29, no. 23, December 69117 Heidelberg (DE) 2009 (2009-12), pages 6321-6334, XP002640895, • Max-Planck-Gesellschaft zur Förderung ISSN: 1098-5549 cited in the application der Wissenschaften e.V. -

Genome-Wide CRISPR Screen Identifies Regulators of Mitogen- Activated Protein Kinase As Suppressors of Liver Tumors in Mice
Genome-Wide CRISPR Screen Identifies Regulators of Mitogen- Activated Protein Kinase as Suppressors of Liver Tumors in Mice The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Song, Chun-qing et al. "Genome-Wide CRISPR Screen Identifies Regulators of Mitogen-Activated Protein Kinase as Suppressors of Liver Tumors in Mice." Gastroenterology 152, 5 (April 2017): 1161-1173 © 2017 AGA Institute As Published http://dx.doi.org/10.1053/j.gastro.2016.12.002 Publisher Elsevier BV Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/121984 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Gastroenterology Manuscript Author . Author Manuscript Author manuscript; available in PMC 2018 October 28. Published in final edited form as: Gastroenterology. 2017 April ; 152(5): 1161–1173.e1. doi:10.1053/j.gastro.2016.12.002. Genome-wide CRISPR Screen Identifies Regulators of MAPK as Suppressors of Liver Tumors in Mice Chun-Qing Song#1, Yingxiang Li#2,3, Haiwei Mou1, Jill Moore2, Angela Park1, Yotsawat Pomyen4, Soren Hough1, Zachary Kennedy1, Andrew Fischer5, Hao Yin6, Daniel G. Anderson6,7,8,9, Darryl Conte Jr1, Lars Zender10, Xin Wei Wang4, Snorri Thorgeirsson4, Zhiping Weng2,3,†, and Wen Xue1,11,† 1RNA Therapeutics Institute, University of Massachusetts Medical School, Worcester, MA 01605 2Program in Bioinformatics and Integrative Biology, University of Massachusetts Medical School, Worcester, MA 01605 3Department of Bioinformatics, School of Life Science and Technology, Tongji University, Shanghai, P. -

(B6;129.Cg-Gt(ROSA)26Sor Tm20(CAG-Ctgf-GFP)Jsd) Were Crossed with Female Foxd1cre/+ Heterozygote Mice 1, and Experimental Mice Were Selected As Foxd1cre/+; Rs26cig/+
Supplemental Information SI Methods Animal studies Heterozygote mice (B6;129.Cg-Gt(ROSA)26Sor tm20(CAG-Ctgf-GFP)Jsd) were crossed with female Foxd1Cre/+ heterozygote mice 1, and experimental mice were selected as Foxd1Cre/+; Rs26CIG/+. In some studies Coll-GFPTg or TCF/Lef:H2B-GFPTg mice or Foxd1Cre/+; Rs26tdTomatoR/+ mice were used as described 2; 3. Left kidneys were subjected to ureteral obstruction using a posterior surgical approach as described 2. In some experiments recombinant mouse DKK1 (0.5mg/kg) or an equal volume of vehicle was administered by daily IP injection. In the in vivo ASO experiment, either specific Lrp6 (TACCTCAATGCGATTT) or scrambled negative control ASO (AACACGTCTATACGC) (30mg/kg) (Exiqon, LNA gapmers) was administered by IP injection on d-1, d1, d4, and d7. In other experiments anti-CTGF domain-IV antibodies (5mg/kg) or control IgG were administered d-1, d1 and d6. All animal experiments were performed under approved IACUC protocols held at the University of Washington and Biogen. Recombinant protein and antibody generation and characterization Human CTGF domain I (sequence Met1 CPDEPAPRCPAGVSLVLDGCGCCRVCAKQLGELCTERDPCDPHKGLFC), domain I+II (sequence Met1CPDEPAPRCPAGVSLVLDGCGCCRVCAKQLGELCTERDPCDPHKGLFCCIFGGT VYRSGESFQSSCKYQCTCLDGAVGCMPLCSMDVRLPSPDCPFPRRVKLPGKCCEE) were cloned and expressed in 293 cells, and purified by Chelating SFF(Ni) Column, tested for single band by SEC and PAGE, and tested for absence of contamination. Domain-IV (sequence GKKCIRTPKISKPIKFELSGCTSMKTYRAKFCGVCTDGRCCTPHRTTTLPVEFKCPDGE VMKKNMMFIKTCACHYNCPGDNDIFESLYYRKMY) was purchased from Peprotech. Mouse or human DKK1 was generated from the coding sequence with some modifications and a tag. Secreted protein was harvested from 293 cells, and purified by nickel column, and tested for activity in a supertopflash (STF) assay 4. DKK1 showed EC50 of 0.69nM for WNT3a-induced WNT signaling in STF cells. -

GIPC Proteins Negatively Modulate Plexind1 Signaling During Vascular Development
RESEARCH ARTICLE GIPC proteins negatively modulate Plexind1 signaling during vascular development Jorge Carretero-Ortega1*, Zinal Chhangawala1, Shane Hunt1, Carlos Narvaez1, Javier Mene´ ndez-Gonza´ lez1, Carl M Gay1, Tomasz Zygmunt1, Xiaochun Li2, Jesu´s Torres-Va´ zquez1* 1Department of Cell Biology, Skirball Institute of Biomolecular Medicine, New York University Langone Medical Center, New York, United States; 2Department of Population Health, New York University School of Medicine, New York, United States Abstract Semaphorins (SEMAs) and their Plexin (PLXN) receptors are central regulators of metazoan cellular communication. SEMA-PLXND1 signaling plays important roles in cardiovascular, nervous, and immune system development, and cancer biology. However, little is known about the molecular mechanisms that modulate SEMA-PLXND1 signaling. As PLXND1 associates with GIPC family endocytic adaptors, we evaluated the requirement for the molecular determinants of their association and PLXND1’s vascular role. Zebrafish that endogenously express a Plxnd1 receptor with a predicted impairment in GIPC binding exhibit low penetrance angiogenesis deficits and antiangiogenic drug hypersensitivity. Moreover, gipc mutant fish show angiogenic impairments that are ameliorated by reducing Plxnd1 signaling. Finally, GIPC depletion potentiates SEMA-PLXND1 signaling in cultured endothelial cells. These findings expand the vascular roles of GIPCs beyond those of the Vascular Endothelial Growth Factor (VEGF)-dependent, proangiogenic GIPC1- Neuropilin 1 complex, recasting GIPCs as negative modulators of antiangiogenic PLXND1 signaling *For correspondence: and suggest that PLXND1 trafficking shapes vascular development. [email protected] (JC- DOI: https://doi.org/10.7554/eLife.30454.001 O); [email protected] (JT-V) Competing interests: The authors declare that no Introduction competing interests exist. Angiogenic sprouting, the formation of new vessels via branching of pre-existing ones, drives most Funding: See page 29 of the life-sustaining expansion of the vascular tree.