Increlex® (Mecasermin); Recombinant Human Insulin-Like Growth Factor-1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protection of Insulin‑Like Growth Factor 1 on Experimental Peripheral Neuropathy in Diabetic Mice
MOLECULAR MEDICINE REPORTS 18: 4577-4586, 2018 Protection of insulin‑like growth factor 1 on experimental peripheral neuropathy in diabetic mice HUA WANG, HAO ZHANG, FUMING CAO, JIAPING LU, JIN TANG, HUIZHI LI, YIYUN ZHANG, BO FENG and ZHAOSHENG TANG Department of Endocrinology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, P.R. China Received December 24, 2017; Accepted July 19, 2018 DOI: 10.3892/mmr.2018.9435 Abstract. The present study investigated whether insulin-like the IGF-1-PPP group compared with the IGF-1 group; however, growth factor-1 (IGF-1) exerts a protective effect against no significant difference was observed in the expression neuropathy in diabetic mice and its potential underlying levels of p-p38 following treatment with IGF-1. The results mechanisms. Mice were divided into four groups: Db/m of the present study demonstrated that IGF-1 may improve (control), db/db (diabetes), IGF-1-treated db/db and neuropathy in diabetic mice. This IGF-1-induced neurotrophic IGF-1-picropodophyllin (PPP)-treated db/db. Behavioral effect may be associated with the increased phosphorylation studies were conducted using the hot plate and von Frey levels of JNK and ERK, not p38; however, it was attenuated by methods at 6 weeks of age prior to treatment. The motor nerve administration of an IGF-1R antagonist. conduction velocity (NCV) of the sciatic nerve was measured using a neurophysiological method at 8 weeks of age. The Introduction alterations in the expression levels of IGF-1 receptor (IGF-1R), c-Jun N-terminal kinase (JNK), extracellular signal-regulated An epidemiological survey demonstrated that the prevalence kinase (ERK), p38 and effect of IGF-1 on the sciatic nerve of diabetes in adults ≥18 years old in China was ≤11.6% (1). -

List of Approved Ndas for Biological Products That Were Deemed to Be Blas on March 23, 2020
List of Approved NDAs for Biological Products That Were Deemed to be BLAs on March 23, 2020 On March 23, 2020, an approved application for a biological product under section 505 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) was deemed to be a license for the biological product under section 351 of the Public Health Service Act (PHS Act) (see section 7002(e)(4)(A) of the Biologics Price Competition and Innovation Act of 2009). To enhance transparency and facilitate planning for the March 23, 2020, transition date, FDA compiled a preliminary list of approved applications for biological products under the FD&C Act that were listed in FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book) and that would be affected by this transition provision. FDA posted this list on the FDA website in December 2018, and periodically updated this list before the March 23, 2020, transition date. The September 2019 update to this preliminary list added certain administratively closed applications related to approved applications for biological products that were on the December 2018 version of this list. The January 2020 update to the preliminary list reflected a change to the definition of “biological product” made by the Further Consolidated Appropriations Act, 2020, which was enacted on December 20, 2019. Section 605 of this Act further amended the definition of a “biological product” in section 351(i) of the PHS Act to remove the parenthetical “(except any chemically synthesized polypeptide)” from the statutory category of “protein.” FDA has provided below a list of each approved application for a biological product under the FD&C Act that was deemed to be a license (i.e., an approved biologics license application (BLA)) for the biological product on March 23, 2020. -

Receptor Activity Modifying Proteins (Ramps) Interact with the VPAC2 Receptor and CRF1 Receptors and Modulate Their Function
British Journal of DOI:10.1111/j.1476-5381.2012.02202.x www.brjpharmacol.org BJP Pharmacology RESEARCH PAPER Correspondence David R Poyner, School of Life and Health Sciences, Aston University, Birmingham, Receptor activity modifying B4 7ET, UK. E-mail: [email protected] ---------------------------------------------------------------- proteins (RAMPs) interact Current address: *Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, 381 with the VPAC2 receptor Royal Parade, Parkville, Victoria 3052 Australia; †Discovery Sciences, AstraZeneca R&D, and CRF1 receptors and Mölndal, Sweden; ‡R&I iMED, AstraZeneca R&D, Mölndal, Sweden. modulate their function ---------------------------------------------------------------- Keywords D Wootten1*, H Lindmark2†, M Kadmiel3, H Willcockson3, KM Caron3, receptor activity-modifying proteins (RAMPs); RAMP1; J Barwell1, T Drmota2‡ and DR Poyner1 RAMP2; RAMP3; VPAC2 receptor; +/- CRF1 receptor; Ramp2 mice; 1 2 School of Life and Health Sciences, Aston University, Birmingham, UK, Department of Lead G-protein coupling; biased Generation, AstraZeneca R&D, Mölndal, Sweden, and 3Department of Cellular and Molecular agonism Physiology, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA ---------------------------------------------------------------- Received 10 July 2012 Revised 15 August 2012 Accepted 28 August 2012 BACKGROUND AND PURPOSE Although it is established that the receptor activity modifying proteins (RAMPs) can interact with a number of GPCRs, little is known about the consequences of these interactions. Here the interaction of RAMPs with the glucagon-like peptide 1 receptor (GLP-1 receptor), the human vasoactive intestinal polypeptide/pituitary AC-activating peptide 2 receptor (VPAC2) and the type 1 corticotrophin releasing factor receptor (CRF1) has been examined. EXPERIMENTAL APPROACH GPCRs were co-transfected with RAMPs in HEK 293S and CHO-K1 cells. -
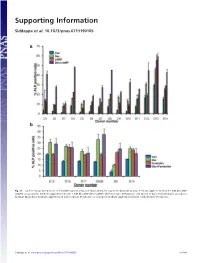
Supporting Information
Supporting Information Siddappa et al. 10.1073/pnas.0711190105 Fig. S1. (a) Percentage ALP-positive cells in hMSCs grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with 1 mM db-cAMP (cAMP), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). (b) Percentage ALP-positive cells grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with forskolin (Forskolin), or osteogenic medium supplemented with forskolin (DexϩForskolin). Siddappa et al. www.pnas.org/cgi/content/short/0711190105 1of10 Fig. S2. hMSCs were grown in basic medium, basic medium supplemented with 1 mM db-cAMP (cAMP), osteogenic medium (Dex), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). Expression was analyzed by qPCR and is expressed as fold induction compared with cells grown in basic medium. The data were analyzed by using two-way ANOVA, and statistical significance is indicated compared with cells grown in basic medium. *, P Ͻ 0.05. Siddappa et al. www.pnas.org/cgi/content/short/0711190105 2of10 Fig. S3. (a) Methylene blue staining of hMSC-seeded scaffolds grown in basic medium (Con) or basic medium supplemented with 1 mM db-cAMP (cAMP) for 4 days. Note the less intensely stained db-cAMP-treated construct, indicating reduced cell numbers. (b) Quantitative Alamar blue assay for cell number analysis. The data were analyzed by using one-way ANOVA followed by Dunnet’s multiple-comparison test. Statistical significance is indicated compared with cells grown in basic medium (Con). *, P Ͻ 0.05 Siddappa et al. www.pnas.org/cgi/content/short/0711190105 3of10 Fig. -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

Alteration in Phosphorylation of P20 Is Associated with Insulin Resistance Yu Wang,1 Aimin Xu,1 Jiming Ye,2 Edward W
Alteration in Phosphorylation of P20 Is Associated With Insulin Resistance Yu Wang,1 Aimin Xu,1 Jiming Ye,2 Edward W. Kraegen,2 Cynthia A. Tse,1 and Garth J.S. Cooper1,3 We have recently identified a small phosphoprotein, P20, insulin action, such as the activation of insulin receptors, as a common intracellular target for insulin and several postreceptor signal transduction, and the glucose trans- of its antagonists, including amylin, epinephrine, and cal- port effector system, have been implicated in this disease citonin gene-related peptide. These hormones elicit phos- (3,4). Defective insulin receptor kinase activity, reduced phorylation of P20 at its different sites, producing three insulin receptor substrate-1 tyrosine phosphorylation, and phosphorylated isoforms: S1 with an isoelectric point (pI) decreased phosphatidylinositol (PI)-3 kinase activity were value of 6.0, S2 with a pI value of 5.9, and S3 with a pI observed in both human type 2 diabetic patients as well as value of 5.6 (FEBS Letters 457:149–152 and 462:25–30, animal models, such as ob/ob mice (5,6). 1999). In the current study, we showed that P20 is one In addition to the intrinsic defects of the insulin receptor of the most abundant phosphoproteins in rat extensor digitorum longus (EDL) muscle. Insulin and amylin an- and postreceptor signaling components, other circulating tagonize each other’s actions in the phosphorylation of factors, such as tumor necrosis factor-␣, leptin, free fatty this protein in rat EDL muscle. Insulin inhibits amylin- acids, and amylin, may also contribute to the pathogenesis evoked phosphorylation of S2 and S3, whereas amylin of insulin resistance (7–11). -

Clinical Policy: Mecasermin (Increlex) Reference Number: ERX.SPA.209 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log
Clinical Policy: Mecasermin (Increlex) Reference Number: ERX.SPA.209 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Mecasermin (Increlex®) is an insulin growth factor-1 (IGF-1) analogue. FDA Approved Indication(s) Increlex is indicated for the treatment of growth failure in children with severe primary IGF-1 deficiency or with growth hormone (GH) gene deletion who have developed neutralizing antibodies to GH. Limitation(s) of use: Increlex is not a substitute to GH for approved GH indications. Policy/Criteria Provider must submit documentation (which may include office chart notes and lab results) supporting that member has met all approval criteria It is the policy of health plans affiliated with Envolve Pharmacy Solutions™ that Increlex is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Severe Primary IGF-1 Deficiency (must meet all): 1. Diagnosis of IGF-1 deficiency growth failure and associated growth failure with one of the following (a or b): a. Severe primary IGF-1 deficiency as defined by all (i through iii): i. Height standard deviation score (SDS) ≤ –3.0; ii. Basal IGF-1 SDS ≤ –3.0; iii. Normal or elevated GH level; b. GH gene deletion with development of neutralizing antibodies to GH; 2. Prescribed by or in consultation with an endocrinologist; 3. Age ≥ 2 and <18 years; 4. At the time of request, member does not have closed epiphyses; 5. Dose does not exceed 0.12 mg/kg twice daily. -

Table of Contents
Therapeutic systems for Insulin-like growth factor-I Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Isabel Schultz aus Dahn Würzburg 2015 Eingereicht bei der Fakultät für Chemie und Pharmazie am Gutachter der schriftlichen Arbeit 1. Gutachter: 2. Gutachter: Prüfer des öffentlichen Promotionskolloquiums 1. Prüfer: 2. Prüfer: 3. Prüfer: Datum des öffentlichen Promotionskolloquiums Doktorurkunde ausgehändigt am TABLE OF CONTENTS TABLE OF CONTENTS SUMMARY ............................................................................... 1 ZUSAMMEMFASSUNG .......................................................... 5 CHAPTER I ............................................................................... 9 DRUG DELIVERY OF INSULIN-LIKE GROWTH FACTOR I CHAPTER II ............................................................................ 45 INSULIN-LIKE GROWTH FACTOR-I AEROSOL FORMULATIONS FOR PULMONARY DELIVERY CHAPTER III ........................................................................... 73 PULMONARY INSULIN-LIKE GROWTH FACTOR I DELIVERY FROM TREHALOSE AND SILK-FIBROIN MICROPARTICLES CHAPTER IV ......................................................................... 113 EXPRESSION OF IGF-I MUTANTS CONCLUSION AND OUTLOOK ......................................... 147 DOCUMENTATION OF AUTHORSHIP ............................. 159 CURRICULUM VITAE ......................................................... 163 ACKNOWLEDGMENTS ..................................................... -

Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature
International Journal of Molecular Sciences Review Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature Michaela Plamper, Bettina Gohlke, Felix Schreiner and Joachim Woelfle * Pediatric Endocrinology and Diabetology Division, Children’s Hospital, University of Bonn, Adenauerallee 119, 53113 Bonn, Germany; [email protected] (M.P.); [email protected] (B.G.); [email protected] (F.S.) * Correspondence: Joachim.Woelfl[email protected]; Tel.: +49-228-2873-3223; Fax: +49-228-2873-3472 Received: 8 March 2018; Accepted: 18 April 2018; Published: 24 April 2018 Abstract: Mutations in the insulin receptor (INSR) gene underlie rare severe INSR-related insulin resistance syndromes (SIR), including insulin resistance type A, Rabson–Mendenhall syndrome and Donohue syndrome (DS), with DS representing the most severe form of insulin resistance. Treatment of these cases is challenging, with the majority of DS patients dying within the first two years of life. rhIGF-I (mecasermin) has been reported to improve metabolic control and increase lifespan in DS patients. A case report and literature review were completed. We present a case involving a male patient with DS, harbouring a homozygous mutation in the INSR gene (c.591delC). Initial rhIGF-I application via BID (twice daily) injection was unsatisfactory, but continuous subcutaneous rhIGF-I infusion via an insulin pump improved weight development and diabetes control (HbA1c decreased from 10 to 7.6%). However, our patient died at 22 months of age during the course of a respiratory infection in in Libya. Currently available data in the literature comprising more than 30 treated patients worldwide seem to support a trial of rhIGF-I in SIR. -

Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile
Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault This information is current as J Immunol 2016; 196:4760-4770; Prepublished online 29 of October 1, 2021. April 2016; doi: 10.4049/jimmunol.1502499 http://www.jimmunol.org/content/196/11/4760 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2016/04/29/jimmunol.150249 Material 9.DCSupplemental References This article cites 65 articles, 18 of which you can access for free at: http://www.jimmunol.org/content/196/11/4760.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 1, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault In order to understand the role of mesenchymal cells (MCs) in the adult thymus, we performed whole transcriptome analyses of primary thymic, bone, and skin MCs. -

Growth Hormone and Insulin-Like Growth Factors I and II Produce
Proc. Natl. Acad. Sci. USA Vol. 82, pp. 8724-8728, December 1985 Medical Sciences Growth hormone and insulin-like growth factors I and II produce distinct alterations in glucose metabolism in 3T3-F442A adipocytes (glucose oxidation/lipid accumulation/somatomedin C/multiplication-stimulating activity/recombinant DNA-derived insulin-like growth factor I) JESSICA SCHWARTZ, CAROL M. FOSTER, AND MARTA S. SATIN Department of Physiology, University of Michigan Medical School, Ann Arbor, MI 48109 Communicated by H. W. Davenport, August 20, 1985 ABSTRACT In 3T3-F442A adipocytes, human growth difficult (9, 10) because the onset of the suppressive effects hormone (hGH) stimulates glucose oxidation in 4 hr. A requires several days and in vitro preparations sensitive to maximal increase is evident at hGH concentrations of 50-100 GH (e.g., adipocytes) are not viable for extended periods. ng/ml and rarely exceeds 50% above control. The stimulation Recently, we reported that cultured 3T3-F442A adipocytes is transient; after 48 hr of incubation with GH, glucose could be used to study the metabolic effects of GH (11). The oxidation is significantly suppressed to 35% below control differentiation of 3T3-F442A fibroblasts to adipocytes has values. In view of the concept that insulin-like growth factors been shown to be GH-dependent (12), although GH is not (IGF) may mediate the effects of GH, we compared the effects required to maintain the adipocytes. We have found that, ofhGH (500 ng/ml) and several preparations ofIGF on glucose once converted, the 3T3 adipocytes are sensitive to both metabolism in 3T3 adipocytes. After 4 hr of incubation, IGF-I stimulatory and suppressive effects of human GH (hGH) on from human plasma stimulated glucose oxidation in a dose- glucose metabolism (11). -

Insulin Products and the Cost of Diabetes Treatment
November 19, 2018 Insulin Products and the Cost of Diabetes Treatment Insulin is a hormone that regulates the storage and use of would involve a consistent insulin level between meals sugar (glucose) by cells in the body. When the pancreas combined with a mealtime level of insulin that has a rapid does not make enough insulin (type 1 diabetes) or it cannot onset and duration of action to match the glucose peak that be used effectively (type 2 diabetes), sugar builds up in the occurs after a meal. The original insulin, also called regular blood. This may lead to serious complications, such as heart insulin, is a short-acting type of product with a duration of disease, stroke, blindness, kidney failure, amputation of action of about 8 hours, making it less suitable for toes, feet, or limbs. Prior to the discovery of insulin providing 24-hour coverage. treatment, type 1 diabetics usually died from this disease. In the late 1930s through the 1950s, regular insulin was There were 23.1 million diagnosed cases of diabetes in the altered by adding substances (protamine and zinc) to gain United States in 2015 according to the Centers for Disease longer action; these are called intermediate-acting insulins. Control and Prevention (CDC). Adding an estimated 7.2 One such advance (neutral protamine Hagedorn, or NPH) million undiagnosed cases brings the total to 30.3 million was patented in 1946 and is still in use today. It allowed for (9.4% of U.S. population). People with type 1 diabetes, the combination of two types of insulin in premixed vials about 5% of U.S.