Neuronal, Stromal, and T-Regulatory Cell Crosstalk in Murine Skeletal Muscle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mutant RAMP2 Causes Primary Open-Angle Glaucoma Via the CRLR-Camp Axis
© American College of Medical Genetics and Genomics ARTICLE Mutant RAMP2 causes primary open-angle glaucoma via the CRLR-cAMP axis Bo Gong, PhD1,2, Houbin Zhang, PhD1, Lulin Huang, PhD1, Yuhong Chen, MD3, Yi Shi, PhD1, Pancy Oi-Sin Tam, MS4, Xianjun Zhu, PhD1, Yi Huang, MD1,5, Bo Lei, PhD6, Periasamy Sundaresan, PhD7, Xi Li, MS1, Linxin Jiang, MS1, Jialiang Yang, MS1, Ying Lin, MS1, Fang Lu, PhD1, Lijia Chen, MRCSEd (Ophth), PhD4, Yuanfeng Li, MS1, Christopher Kai-Shun Leung, MD4, Xiaoxin Guo, MS1, Shanshan Zhang, MS1, Guo Huang, MS1, Yaqi Wu, MS1, Tongdan Zhou, MS1, Ping Shuai, PhD1, Clement Chee-Yung Tham, FRCOphth4, Nicole Weisschuh, PhD8, Subbaiah Ramasamy Krishnadas, MD7, Christian Mardin, MD9, André Reis, PhD10, Jiyun Yang, MD1, Lin Zhang, PhD1,3, Yu Zhou, PhD1, Ziyan Wang, PhD1, Chao Qu, MD11, Peter X. Shaw, PhD12, Chi-Pui Pang, DPhil4, Xinghuai Sun, MD3, Weiquan Zhu, MD5, Dean Yaw Li, MD5, Francesca Pasutto, PhD10 and Zhenglin Yang, MD, PhD1,2 Purpose: Primary open-angle glaucoma (POAG) is the leading 4763 POAG patients, whereas no variants were detected in any cause of irreversible blindness worldwide and mutations in known exon of RAMP2 in 10,953 control individuals. Mutant RAMP2s genes can only explain 5–6% of POAG. This study was conducted aggregated in transfected cells and resulted in damage to the AM- to identify novel POAG-causing genes and explore the pathogenesis RAMP2/CRLR-cAMP signaling pathway. Ablation of one Ramp2 of this disease. allele led to cAMP reduction and retinal ganglion cell death in mice. Methods: Exome sequencing was performed in a Han Chinese Conclusion: This study demonstrated that disruption of RAMP2/ cohort comprising 398 sporadic cases with POAG and 2010 CRLR-cAMP axis could cause POAG and identified a potential controls, followed by replication studies by Sanger sequencing. -

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -

Protection of Insulin‑Like Growth Factor 1 on Experimental Peripheral Neuropathy in Diabetic Mice
MOLECULAR MEDICINE REPORTS 18: 4577-4586, 2018 Protection of insulin‑like growth factor 1 on experimental peripheral neuropathy in diabetic mice HUA WANG, HAO ZHANG, FUMING CAO, JIAPING LU, JIN TANG, HUIZHI LI, YIYUN ZHANG, BO FENG and ZHAOSHENG TANG Department of Endocrinology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, P.R. China Received December 24, 2017; Accepted July 19, 2018 DOI: 10.3892/mmr.2018.9435 Abstract. The present study investigated whether insulin-like the IGF-1-PPP group compared with the IGF-1 group; however, growth factor-1 (IGF-1) exerts a protective effect against no significant difference was observed in the expression neuropathy in diabetic mice and its potential underlying levels of p-p38 following treatment with IGF-1. The results mechanisms. Mice were divided into four groups: Db/m of the present study demonstrated that IGF-1 may improve (control), db/db (diabetes), IGF-1-treated db/db and neuropathy in diabetic mice. This IGF-1-induced neurotrophic IGF-1-picropodophyllin (PPP)-treated db/db. Behavioral effect may be associated with the increased phosphorylation studies were conducted using the hot plate and von Frey levels of JNK and ERK, not p38; however, it was attenuated by methods at 6 weeks of age prior to treatment. The motor nerve administration of an IGF-1R antagonist. conduction velocity (NCV) of the sciatic nerve was measured using a neurophysiological method at 8 weeks of age. The Introduction alterations in the expression levels of IGF-1 receptor (IGF-1R), c-Jun N-terminal kinase (JNK), extracellular signal-regulated An epidemiological survey demonstrated that the prevalence kinase (ERK), p38 and effect of IGF-1 on the sciatic nerve of diabetes in adults ≥18 years old in China was ≤11.6% (1). -

Multifaceted Physiological Roles of Adiponectin in Inflammation And
International Journal of Molecular Sciences Review Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases Hyung Muk Choi 1, Hari Madhuri Doss 1,2 and Kyoung Soo Kim 1,2,* 1 Department of Clinical Pharmacology and Therapeutics, Kyung Hee University School of Medicine, Seoul 02447, Korea; [email protected] (H.M.C.); [email protected] (H.M.D.) 2 East-West Bone & Joint Disease Research Institute, Kyung Hee University Hospital at Gangdong, Gandong-gu, Seoul 02447, Korea * Correspondence: [email protected]; Tel.: +82-2-961-9619 Received: 3 January 2020; Accepted: 10 February 2020; Published: 12 February 2020 Abstract: Adiponectin is the richest adipokine in human plasma, and it is mainly secreted from white adipose tissue. Adiponectin circulates in blood as high-molecular, middle-molecular, and low-molecular weight isoforms. Numerous studies have demonstrated its insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects. Additionally, decreased serum levels of adiponectin is associated with chronic inflammation of metabolic disorders including Type 2 diabetes, obesity, and atherosclerosis. However, recent studies showed that adiponectin could have pro-inflammatory roles in patients with autoimmune diseases. In particular, its high serum level was positively associated with inflammation severity and pathological progression in rheumatoid arthritis, chronic kidney disease, and inflammatory bowel disease. Thus, adiponectin seems to have both pro-inflammatory and anti-inflammatory effects. This indirectly indicates that adiponectin has different physiological roles according to an isoform and effector tissue. Knowledge on the specific functions of isoforms would help develop potential anti-inflammatory therapeutics to target specific adiponectin isoforms against metabolic disorders and autoimmune diseases. -

Neuromedin U Directly Stimulates Growth of Cultured Rat Calvarial Osteoblast-Like Cells Acting Via the NMU Receptor 2 Isoform
363-368 1/8/08 15:53 Page 363 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 22: 363-368, 2008 363 Neuromedin U directly stimulates growth of cultured rat calvarial osteoblast-like cells acting via the NMU receptor 2 isoform MARCIN RUCINSKI, AGNIESZKA ZIOLKOWSKA, MARIANNA TYCZEWSKA, MARTA SZYSZKA and LUDWIK K. MALENDOWICZ Department of Histology and Embryology, Poznan University of Medical Sciences, 6 Swiecicki St., 60-781 Poznan, Poland Received April 4, 2008; Accepted June 2, 2008 DOI: 10.3892/ijmm_00000031 Abstract. The neuromedin U (NMU) system is composed of nervous system. Among others, peptides involved in regulation NMU, neuromedin S (NMS) and their receptors NMUR1 and of energy homeostasis belong to this group of compounds NMUR2. This system is involved in the regulation of energy (1-3), and the best recognised is leptin, an adipocyte-derived homeostasis, neuroendocrine functions, immune response, anorexigenic hormone, which plays a role in regulating bone circadian rhythm and spermatogenesis. The present study formation. Acting directly this pleiotropic cytokine exerts a aimed to investigate the possible role of the NMU system in stimulatory effect on bone formation. While acting through regulating functions of cultured rat calvarial osteoblast-like the central nervous system (CNS) leptin suppresses bone (ROB) cells. By using QPCR, high expression of NMU formation (4-10). Moreover, OB-Rb mRNA is expressed in mRNA was found in freshly isolated ROB cells while after 7, osteoblasts, and in vitro leptin enhances their proliferation 14, and 21 days of culture, expression of the studied gene and has no effect on osteocalcin and osteopontin production by was very low. -
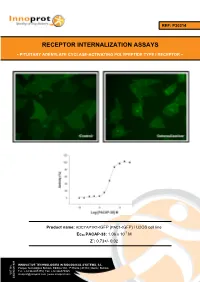
Receptor Internalization Assays
REF: P30214 RECEPTOR INTERNALIZATION ASSAYS - PITUITARY ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE TYPE I RECEPTOR - Product name: ADCYAP1R1-tGFP (PAC1-tGFP) / U2OS cell line -7 Ec50 PACAP-38: 1.06 x 10 M Z´: 0.73+/- 0.02 INNOVATIVE TECHNOLOGIES IN BIOLOGICAL SYSTEMS, S.L. Parque Tecnológico Bizkaia, Edifício 502, 1ª Planta | 48160 | Derio | Bizkaia Tel.: +34 944005355 | Fax: +34 946579925 VAT No. [email protected] | www.innoprot.com ESB95481909 Product Name: ADCYAP1R1-tGFP_U2OS Reference: P30214 Rep. Official Full Name: Pituitary adenylate cyclase- activating polypeptide type I receptor DNA Accession Number: Gene Bank AY366498 Host Cell: U2OS References: P30214: 2 vials of 3 x 106 proliferative cells P30214-DA: 1 vial of 2 x 106 division-arrested cells Storage: Liquid Nitrogen Assay Briefly description About ADCYAP1R1 Each vial of ADCYAP1R1 Internalization Assay Pituitary adenylate cyclase-activating Cell Line contains U2OS cells stably expressing polypeptide type I receptor, also known as human Pituitary adenylate cyclase-activating PAC1 is a protein that in humans is encoded by polypeptide type I receptor tagged in the N- the ADCYAP1R1 gene. ADCYAP1R1 is a terminus with tGFP protein. membrane-associated protein and shares significant homology with members of the Innoprot’s ADCYAP1R1-tGFP Internalization glucagon/secretin receptor family. This receptor Assay Cell Line has been designed to assay binds pituitary adenylate cyclase activating potential agonists/ antagonists against peptide (PACAP) mediating several biological ADCYAP1R1, modulating its activation and the activities and it is positively coupled to following redistribution process inside the cells. adenylate cyclase. This cell line will allow the image analysis of the stimuli induced by the compounds. -

Receptor Activity Modifying Proteins (Ramps) Interact with the VPAC2 Receptor and CRF1 Receptors and Modulate Their Function
British Journal of DOI:10.1111/j.1476-5381.2012.02202.x www.brjpharmacol.org BJP Pharmacology RESEARCH PAPER Correspondence David R Poyner, School of Life and Health Sciences, Aston University, Birmingham, Receptor activity modifying B4 7ET, UK. E-mail: [email protected] ---------------------------------------------------------------- proteins (RAMPs) interact Current address: *Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, 381 with the VPAC2 receptor Royal Parade, Parkville, Victoria 3052 Australia; †Discovery Sciences, AstraZeneca R&D, and CRF1 receptors and Mölndal, Sweden; ‡R&I iMED, AstraZeneca R&D, Mölndal, Sweden. modulate their function ---------------------------------------------------------------- Keywords D Wootten1*, H Lindmark2†, M Kadmiel3, H Willcockson3, KM Caron3, receptor activity-modifying proteins (RAMPs); RAMP1; J Barwell1, T Drmota2‡ and DR Poyner1 RAMP2; RAMP3; VPAC2 receptor; +/- CRF1 receptor; Ramp2 mice; 1 2 School of Life and Health Sciences, Aston University, Birmingham, UK, Department of Lead G-protein coupling; biased Generation, AstraZeneca R&D, Mölndal, Sweden, and 3Department of Cellular and Molecular agonism Physiology, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA ---------------------------------------------------------------- Received 10 July 2012 Revised 15 August 2012 Accepted 28 August 2012 BACKGROUND AND PURPOSE Although it is established that the receptor activity modifying proteins (RAMPs) can interact with a number of GPCRs, little is known about the consequences of these interactions. Here the interaction of RAMPs with the glucagon-like peptide 1 receptor (GLP-1 receptor), the human vasoactive intestinal polypeptide/pituitary AC-activating peptide 2 receptor (VPAC2) and the type 1 corticotrophin releasing factor receptor (CRF1) has been examined. EXPERIMENTAL APPROACH GPCRs were co-transfected with RAMPs in HEK 293S and CHO-K1 cells. -

Adiporon, an Orally Active, Synthetic Agonist of Adipor1 and Adipor2 Receptors Has Gastroprotective Effect in Experimentally Induced Gastric Ulcers in Mice
molecules Article AdipoRon, an Orally Active, Synthetic Agonist of AdipoR1 and AdipoR2 Receptors Has Gastroprotective Effect in Experimentally Induced Gastric Ulcers in Mice Hubert Zatorski 1,2, Maciej Salaga 1 , Marta Zieli ´nska 1, Kinga Majchrzak 1, Agata Binienda 1 , Radzisław Kordek 3, Ewa Małecka-Panas 2 and Jakub Fichna 1,4,* 1 Department of Biochemistry, Medical University of Lodz, 92-215 Lodz, Poland; [email protected] (H.Z.); [email protected] (M.S.); [email protected] (M.Z.); [email protected] (K.M.); [email protected] (A.B.) 2 Department of Digestive Tract Diseases, Medical University of Lodz, 93-281 Lodz, Poland; [email protected] 3 Department of Pathology, Medical University of Lodz, 92-215 Lodz, Poland; [email protected] 4 Department of Biochemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland * Correspondence: jakub.fi[email protected]; Tel.: +48-42-272-57-07 Citation: Zatorski, H.; Salaga, M.; Abstract: Introduction: Adiponectin is a hormone secreted by adipocytes, which exhibits insulin- Zieli´nska,M.; Majchrzak, K.; sensitizing and anti-inflammatory properties and acts through adiponectin receptors: AdipoR1 and Binienda, A.; Kordek, R.; AdipoR2. The aim of the study was to evaluate whether activation of adiponectin receptors AdipoR1 Małecka-Panas, E.; Fichna, J. and AdipoR2 with an orally active agonist AdipoRon has gastroprotective effect and to investigate AdipoRon, an Orally Active, the possible underlying mechanism. Methods: We used two well-established mouse models of Synthetic Agonist of AdipoR1 and gastric ulcer (GU) induced by oral administration of EtOH (80% solution in water) or diclofenac AdipoR2 Receptors Has (30 mg/kg, p.o.). -

In the Porcine Pituitary During the Oestrous Cycle Marta Kiezun, Anna Maleszka, Nina Smolinska, Anna Nitkiewicz and Tadeusz Kaminski*
Kiezun et al. Reproductive Biology and Endocrinology 2013, 11:18 http://www.rbej.com/content/11/1/18 RESEARCH Open Access Expression of adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) in the porcine pituitary during the oestrous cycle Marta Kiezun, Anna Maleszka, Nina Smolinska, Anna Nitkiewicz and Tadeusz Kaminski* Abstract Background: Adiponectin, protein secreted mainly by white adipose tissue, is an important factor linking the regulation of metabolic homeostasis and reproductive processes. The biological activity of the hormone is mediated via two distinct receptors, termed adiponectin receptor 1(AdipoR1) and adiponectin receptor 2 (AdipoR2). The present study analyzed mRNA and protein expression of AdipoR1 and AdipoR2 in the anterior (AP) and posterior (NP) pituitary of cyclic pigs. Methods: The total of 20 animals was assigned to one of four experimental groups (n = 5 per group) as follows: days 2–3 (early-luteal phase), 10–12 (mid-luteal phase), 14–16 (late-luteal phase), 17–19 (follicular phase) of the oestrous cycle. mRNA and protein expression were analyzed using real-time PCR and Western Blot methods, respectively. Results: The lowest AdipoR1 gene expression was detected in AP on days 10–12 relative to days 2–3and14–16 (p < 0.05). In NP, AdipoR1 mRNA levels were elevated on days 10–12 and 14–16 (p < 0.05). AdipoR2 gene expression in AP was the lowest on days 10–12, and an expression peak occurred on days 2–3 (p < 0.05). In NP, the lowest (p < 0.05) expression of AdipoR2 mRNA was noted on days 17–19. The AdipoR1 protein content in AP was the lowest on days 17–19 (p < 0.05), while in NP the variations in protein expression levels during the oestrous cycle were negligible. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -

Effect of Neuromedin U on Allergic Airway Inflammation in an Asthma Model (Review)
EXPERIMENTAL AND THERAPEUTIC MEDICINE 19: 809-816, 2020 Effect of neuromedin U on allergic airway inflammation in an asthma model (Review) XIAOJIE REN1,2, FANG DONG1,2, YUERONG ZHUANG1,2, YONG WANG1,2 and WUHUA MA1,2 1Guangzhou University of Chinese Medicine; 2Department of Anaesthesiology, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong 510405, P.R. China Received February 19, 2019; Accepted November 14, 2019 DOI: 10.3892/etm.2019.8283 Abstract. Asthma is a major inflammatory airway disease Contents with high incidence and mortality rates. The Global Initiative for Asthma released a report called ‘The Global 1. Introduction Burden of Asthma’ in 2004. However, the specific patho- 2. Overview of asthma genesis of asthma remains unclear. An increasing number of 3. Biology of neuromedin U studies have demonstrated that neuromedin U (NMU) plays 4. NMU acts as a multifunctional neuropeptide in the a pleiotropic role in the pathogenesis of asthma. NMU is pathogenesis of asthma a highly structurally conserved neuropeptide that was first 5. Clinical implications of NMU involvement in asthma purified from porcine spinal cord and named for its contrac- 6. Conclusion tile effect on the rat uterus. NMU amplifies type 2 innate lymphoid cell (ILC2)‑driven allergic lung inflammation. The NMU receptors (NMURs), designated as NMUR1 and 1. Introduction NMUR2, belong to the G protein‑coupled receptor family. NMUR1 has also been found in immune cells, including Asthma is a heterogeneous disease that is usually char- ILC2s, mast cells and eosinophils. In view of the important acterized by chronic airway inflammation with airway roles of NMU in the pathogenesis of asthma, the present hyper‑ responsiveness, airway remodelling and disordered review evaluates the potential mechanisms underlying the mucosal immunity (1‑3). -

Alteration in Phosphorylation of P20 Is Associated with Insulin Resistance Yu Wang,1 Aimin Xu,1 Jiming Ye,2 Edward W
Alteration in Phosphorylation of P20 Is Associated With Insulin Resistance Yu Wang,1 Aimin Xu,1 Jiming Ye,2 Edward W. Kraegen,2 Cynthia A. Tse,1 and Garth J.S. Cooper1,3 We have recently identified a small phosphoprotein, P20, insulin action, such as the activation of insulin receptors, as a common intracellular target for insulin and several postreceptor signal transduction, and the glucose trans- of its antagonists, including amylin, epinephrine, and cal- port effector system, have been implicated in this disease citonin gene-related peptide. These hormones elicit phos- (3,4). Defective insulin receptor kinase activity, reduced phorylation of P20 at its different sites, producing three insulin receptor substrate-1 tyrosine phosphorylation, and phosphorylated isoforms: S1 with an isoelectric point (pI) decreased phosphatidylinositol (PI)-3 kinase activity were value of 6.0, S2 with a pI value of 5.9, and S3 with a pI observed in both human type 2 diabetic patients as well as value of 5.6 (FEBS Letters 457:149–152 and 462:25–30, animal models, such as ob/ob mice (5,6). 1999). In the current study, we showed that P20 is one In addition to the intrinsic defects of the insulin receptor of the most abundant phosphoproteins in rat extensor digitorum longus (EDL) muscle. Insulin and amylin an- and postreceptor signaling components, other circulating tagonize each other’s actions in the phosphorylation of factors, such as tumor necrosis factor-␣, leptin, free fatty this protein in rat EDL muscle. Insulin inhibits amylin- acids, and amylin, may also contribute to the pathogenesis evoked phosphorylation of S2 and S3, whereas amylin of insulin resistance (7–11).