Disorders of Magnesium Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dysmagnesemia in Covid-19 Cohort Patients: Prevalence and Associated Factors
Magnesium Research 2020; 33 (4): 114-122 ORIGINAL ARTICLE Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors Didier Quilliot1, Olivier Bonsack1, Roland Jaussaud2, Andre´ Mazur3 1 Transversal Nutrition Unit and; 2 Internal Medicine and Clinical Immunology. Nancy University Hospital, University of Lorraine, France; 3 Universite´ Clermont Auvergne, INRAE, UNH, Unite´ de Nutrition Humaine, Clermont-Ferrand, France Correspondence <[email protected]> Abstract. Hypomagnesemia and hypermagnesemia could have serious implications and possibly lead to progress from a mild form to a severe outcome of Covid-19. Susceptibility of subjects with low magnesium status to develop and enhance this infection is possible. There is little data on the magnesium status of patients with Covid-19 with different degrees of severity. This study was conducted to evaluate prevalence of dysmagnesemia in a prospective Covid-19 cohort study according to the severity of the clinical manifestations and to identify factors associated. Serum magnesium was measured in 300 of 549 patients admitted to the hospital due to severe Covid-19. According to the WHO guidelines, patients were classified as moderate, severe, or critical. 48% patients had a magnesemia below 0.75 mmol/L (defined as magnesium deficiency) including 13% with a marked hypomagnesemia (<0.65 mmol/L). 9.6% had values equal to or higher than 0.95 mmol/L. Serum magnesium concentrations were significantly lower in female than in male (0.73 Æ 0.12 vs 0.80 Æ 0.13 mmol/L), whereas the sex ratio M/F was higher in severe and critical form (p<0.001). In a bivariate analysis, the risk of magnesium deficiency was significantly and negatively associated with infection severity (p<0.001), sex ratio (M/F, p<0.001), oxygenotherapy (p<0.001), stay in critical care unit (p=0.028), and positively with nephropathy (p=0.026). -

Hypokalaemia in a Woman with Eating Disorder
Grand Rounds Vol 11 pages 53–55 Specialities: Acute Medicine; Nephrology; Psychiatry Article Type: Case Report DOI: 10.1102/1470-5206.2011.0013 ß 2011 e-MED Ltd Hypokalaemia in a woman with eating disorder Zachary Z. Brenera, Boris Medvedovskya, James F. Winchestera and Michael Bergmanb aDivision of Nephrology, Department of Medicine, Beth Israel Medical Center, Albert Einstein School of Medicine of Yeshiva University, New York, USA; bDepartment of Medicine, Campus Golda, Rabin Medical Center, Petah-Tikva, Tel-Aviv University, Israel Corresponding address: Dr Zachary Z. Brener, 350 E. 17th St., Division of Nephrology, Beth Israel Medical Center, New York, NY 10003, USA. Email: [email protected] Date accepted for publication 13 April 2011 Abstract Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. A concealed diuretic abuse should be suspected, especially in young women with eating disorders. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to early and accurate diagnosis. Keywords Hypokalaemia; eating disorders; diuretics. Introduction Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. After the exclusion of the most obvious causes, a concealed diuretic abuse associated with or without surreptitious vomiting and laxative abuse should be suspected, especially in young women concerned with their body image. A conclusive diagnosis may be difficult as such patients often vigorously deny diuretic intake[1]. Also, only a minority of patients with eating disorders (approximately 6%) abuse diuretics[2–4]. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to an early and accurate diagnosis. -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -
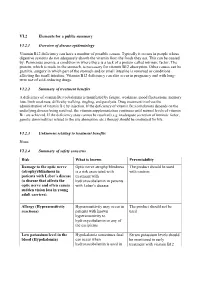
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Increased Mortality Associated with Hypermagnesemia in Severe COVID-19 Illness
Original Investigation Increased Mortality Associated with Hypermagnesemia in Severe COVID-19 Illness Jacob S. Stevens 1,2, Andrew A. Moses1, Thomas L. Nickolas1,2, Syed Ali Husain1,2, and Sumit Mohan1,2,3 Key Points Hypermagnesemia is common in patients admitted with coronavirus disease 2019. The development of hypermagnesemia in coronavirus disease 2019 is associated with renal failure and markers of high cell turnover. In adjusted models, patients who develop hypermagnesemia have an increased risk of mortality. Abstract Background Although electrolyte abnormalities are common among patients with COVID-19, very little has been reported on magnesium homeostasis in these patients. Here we report the incidence of hypermagnesemia, and its association with outcomes among patients admitted with COVID-19. Methods We retrospectively identified all patients with a positive test result for SARS-CoV-2who were admitted to a large quaternary care center in New York City in spring 2020. Details of the patients’ demographics and hospital course were obtained retrospectively from medical records. Patients were defined as having hypermagnesemia if their median magnesium over the course of their hospitalization was .2.4 mg/dl. Results A total of 1685 patients hospitalized with COVID-19 had their magnesium levels checked during their hospitalization, and were included in the final study cohort, among whom 355 (21%) had hypermagnesemia. Patients who were hypermagnesemic had a higher incidence of shock requiring pressors (35% vs 27%, P,0.01), respiratory failure requiring mechanical ventilation (28% vs 21%, P50.01), AKI (65% vs 50%, P,0.001), and AKI severe enough to require renal replacement therapy (18% vs 5%, P,0.001). -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -

Early-Onset Neonatal Hyperkalemia Associated with Maternal
Tanaka et al. BMC Pediatrics (2018) 18:55 https://doi.org/10.1186/s12887-018-1048-4 CASEREPORT Open Access Early-onset neonatal hyperkalemia associated with maternal hypermagnesemia: a case report Kenichi Tanaka1, Hiroko Mori1, Rieko Sakamoto2, Shirou Matsumoto2, Hiroshi Mitsubuchi1, Kimitoshi Nakamura2 and Masanori Iwai1* Abstract Background: Neonatal nonoliguric hyperkalemia (NOHK) is a metabolic abnormality that occurs in extremely premature neonates at approximately 24 h after birth and is mainly due to the immature functioning of the sodium (Na+)/potassium (K+) pump. Magnesium sulfate is frequently used in obstetrical practice to prevent preterm labor and to treat preeclampsia; this medication can also cause hypermagnesemia and hyperkalemia by a mechanism that is different from that of NOHK. Herein, we report the first case of very early-onset neonatal hyperkalemia induced by maternal hypermagnesemia. Case presentation: A neonate born at 32 weeks of gestation developed hyperkalemia (K+ 6.4 mmol/L) 2 h after birth. The neonate’s blood potassium concentration reached 7.0 mmol/L 4 h after birth, despite good urine output. The neonate and his mother had severe hypermagnesemia caused by intravenous infusion of magnesium sulfate given for tocolysis due to pre-term labor. Conclusion: The early-onset hyperkalemia may have been caused by the accumulation of potassium ions transported through the placenta, the shift of potassium ions from the intracellular to the extracellular space in the infant due to the malfunctioning of the Na+/K+ pump and the inhibition of renal distal tube potassium ion secretion, there is a possibility that these mechanisms were induced by maternal and fetal hypermagnesemia after maternal magnesium sulfate administration. -

Electrolyte Disorders and Arrhythmogenesis
Cardiology Journal 2011, Vol. 18, No. 3, pp. 233–245 Copyright © 2011 Via Medica REVIEW ARTICLE ISSN 1897–5593 Electrolyte disorders and arrhythmogenesis Nabil El-Sherif1, Gioia Turitto2 1State University of NY, Downstate Medical Center and NY Harbor VA Healthcare System, Brooklyn, NY, USA 2Methodist University Hospital, Brooklyn, NY, USA Abstract Electrolyte disorders can alter cardiac ionic currents kinetics and depending on the changes can promote proarrhythmic or antiarrhythmic effects. The present report reviews the mecha- nisms, electrophysiolgical (EP), electrocardiographic (ECG), and clinical consequences of elec- trolyte disorders. Potassium (K+) is the most abundent intracellular cation and hypokalemia is the most commont electrolyte abnormality encountered in clinical practice. The most signifcant ECG manifestation of hypokalemia is a prominent U wave. Several cardiac and + non cardiac drugs are known to suppress the HERG K channel and hence the IK, and especially in the presence of hypokalemia, can result in prolonged action potential duration and QT interval, QTU alternans, early afterdepolarizations, and torsade de pointes ventricu- lar tachyarrythmia (TdP VT). Hyperkalemia affects up to 8% of hospitalized patients mainly in the setting of compromised renal function. The ECG manifestation of hyperkalemia de- pends on serum K+ level. At 5.5–7.0 mmol/L K+, tall peaked, narrow-based T waves are seen. At > 10.0 mmol/L K+, sinus arrest, marked intraventricular conduction delay, ventricular techycardia, and ventricular fibrillation can develop. Isolated abnormalities of extracellular calcium (Ca++) produce clinically significant EP effects only when they are extreme in either direction. Hypocalcemia, frequently seen in the setting of chronic renal insufficiency, results in prolonged ST segment and QT interval while hypercalcemia, usually seen with hyperparathy- roidism, results in shortening of both intervals. -

Front Matter (PDF)
HIGH RATE OF SUCCESS IN AN NIH- STUDY of hypertensive patients- highest percentage - remained on itial therapy 83% with NORVASC#{174}(amlodipi besylate) after 4 years; nearly all pati were on the 5-mg starting dose’ LOW RATE DISCONTINUATION ONLY 1.5%of patients in placebo studies (n= 1730) discontinued due to adverse effects2 PROVEN No negative inotropic effects clinical doses in hemodyna m ic stud 2* No clinically significant effect on cardiac conduction or heart rate2 *S1IflH,jr fiernodynamic findings, hcvever fiave been agents possessing significant neqafive notropic ebecto and 10-mg tablets Once-Daily NORV (ambdipine EFFICACY AND SAFETY THAT’S EASY TO WITH Br$e(Summary NORVASC(amlodlpine besylate) Tablets For Oral Use CONTRAINDICATIONS: NORVASC is contraindicated in patients with known sensitivity to arniodipirre. In hypertension WARNINGS: Increased Angina and/or MyOcardIal Infarction: Rarely, patients, particulady those with severe obstruc5ve coronary artery disease, have developed documented increased frequency,durationand/or severity of angina or acute myocardial infarction on starhng cafrium channel blocker therapy or attire time of dosage increase. The mechanism of this effect has not been elucidated. PRECAUTIONS: General: Since the vasoditation induced by NORVASC is gradual in onset, acute hypotension has rarely been reported after oraladministrationof NORVASC. Nonethetuss, caution should be exercised when admin- or angina, convenient istering NORVASC as with any other penpherio vasodilator particularly in pahentswithsevere -

Women'shealthreport
Issue 4, 2014 Women’sHealthREPORT A QuaRTERLY PubLicatiON OF WOMEN’S HEALth diETEtic PRacticE GROup MICRONUTRIENTS AND THE OLDER ADULT – Part 1: Micronutrients of Importance to Older Adults By Vijaya Jain, MS, MSc, RD, CDN among older adults. A decreased energy intake associated with advancing age has important implications in terms of nutrient intake among older adults, including protein and micronutrients. Suboptimal intake for several micronutrients is prevalent among older adults. INTRODUCTION Increasing life expectancy has led to a steady rise in the number of older persons globally. In 2012, the number of older Americans aged 65 years and older had reached 43.1 million (1). As many of these older Americans continue to remain healthy and active well into their eighties and even nineties, and have better medi- cal management of chronic diseases and their comorbidities, the size of the older population is projected to double between 2000 and 2030 (2). According to the US Bureau of the Census, the size of the population age 85 and over is projected to be 7 Note from Chair: Collaboration is essential to the success of both million by 2020 (1). The baby-boomer generation will reach age individuals and organizations. It is has been a personal goal of mine 65 between 2010 and 2030, meaning that by 2030, one in five to see the Women’s Health DPG work with other DPGs and related American citizens will be older adults (1), and two out of every health organizations. In recent years, we have enhanced our mission three older Americans will have multiple chronic conditions (3).