Electrolyte Disorders and Arrhythmogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dysmagnesemia in Covid-19 Cohort Patients: Prevalence and Associated Factors
Magnesium Research 2020; 33 (4): 114-122 ORIGINAL ARTICLE Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors Didier Quilliot1, Olivier Bonsack1, Roland Jaussaud2, Andre´ Mazur3 1 Transversal Nutrition Unit and; 2 Internal Medicine and Clinical Immunology. Nancy University Hospital, University of Lorraine, France; 3 Universite´ Clermont Auvergne, INRAE, UNH, Unite´ de Nutrition Humaine, Clermont-Ferrand, France Correspondence <[email protected]> Abstract. Hypomagnesemia and hypermagnesemia could have serious implications and possibly lead to progress from a mild form to a severe outcome of Covid-19. Susceptibility of subjects with low magnesium status to develop and enhance this infection is possible. There is little data on the magnesium status of patients with Covid-19 with different degrees of severity. This study was conducted to evaluate prevalence of dysmagnesemia in a prospective Covid-19 cohort study according to the severity of the clinical manifestations and to identify factors associated. Serum magnesium was measured in 300 of 549 patients admitted to the hospital due to severe Covid-19. According to the WHO guidelines, patients were classified as moderate, severe, or critical. 48% patients had a magnesemia below 0.75 mmol/L (defined as magnesium deficiency) including 13% with a marked hypomagnesemia (<0.65 mmol/L). 9.6% had values equal to or higher than 0.95 mmol/L. Serum magnesium concentrations were significantly lower in female than in male (0.73 Æ 0.12 vs 0.80 Æ 0.13 mmol/L), whereas the sex ratio M/F was higher in severe and critical form (p<0.001). In a bivariate analysis, the risk of magnesium deficiency was significantly and negatively associated with infection severity (p<0.001), sex ratio (M/F, p<0.001), oxygenotherapy (p<0.001), stay in critical care unit (p=0.028), and positively with nephropathy (p=0.026). -

7.016 Introductory Biology Fall 2018
7.016: Fall 2018: MIT 7.016 Recitation 15 – Fall 2018 (Note: The recitation summary should NOT be regarded as the substitute for lectures) (This material is COPYRIGHT protected.) Summary of Lecture 22 (11/2): Neurons and action potentials: Ions can move across membranes through pumps and channels. Integral membrane proteins like these cross the membrane via transmembrane domains. Pumps are ATPases that set up the concentration gradients of ions across cell membranes, such that K+ is high inside cells and other ions (such as Cl–, Na+, and Ca2+) are high outside cells. A membrane potential is only set up by ions that move freely across the membrane through open channels, creating one side of the membrane that is more positive relative to the other side. This movement of ions does not dissipate the concentration gradient because the number of ions that move to generate a membrane potential is very small compared to the number of ions that need to be pumped to create a concentration gradient. Most cell membranes only contain open K+ channels and thus only K+ flows freely across membranes through channels; K+ is high inside, causing it to flow outside, giving the inside of cells a negative membrane potential. Ions can move across the membrane through open ion channels. Two forces act to dictate this movement – the concentration gradient and the electrical gradient. Ions move down their concentration gradient through channels, and ions move towards the side of the membrane that harbors the opposite charge. Neurons are the cells of your nervous system that make connections with each other to transmit impulse/ signals. -

1 Fluid and Elect. Disorders of Serum Sodium Concentration
DISORDERS OF SERUM SODIUM CONCENTRATION Bruce M. Tune, M.D. Stanford, California Regulation of Sodium and Water Excretion Sodium: glomerular filtration, aldosterone, atrial natriuretic factors, in response to the following stimuli. 1. Reabsorption: hypovolemia, decreased cardiac output, decreased renal blood flow. 2. Excretion: hypervolemia (Also caused by adrenal insufficiency, renal tubular disease, and diuretic drugs.) Water: antidiuretic honnone (serum osmolality, effective vascular volume), renal solute excretion. 1. Antidiuresis: hyperosmolality, hypovolemia, decreased cardiac output. 2. Diuresis: hypoosmolality, hypervolemia ~ natriuresis. Physiologic changes in renal salt and water excretion are more likely to favor conservation of normal vascular volume than nonnal osmolality, and may therefore lead to abnormalities of serum sodium concentration. Most commonly, 1. Hypovolemia -7 salt and water retention. 2. Hypervolemia -7 salt and water excretion. • HYFERNATREMIA Clinical Senini:: Sodium excess: salt-poisoning, hypertonic saline enemas Primary water deficit: chronic dehydration (as in diabetes insipidus) Mechanism: Dehydration ~ renal sodium retention, even during hypernatremia Rapid correction of hypernatremia can cause brain swelling - Management: Slow correction -- without rapid administration of free water (except in nephrogenic or untreated central diabetes insipidus) HYPONA1REMIAS Isosmolar A. Factitious: hyperlipidemia (lriglyceride-plus-plasma water volume). B. Other solutes: hyperglycemia, radiocontrast agents,. mannitol. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Genetic Alteration of the Metal/Redox Modulation of Cav3.2 T-Type
Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory To cite this version: Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory. Genetic alteration of the metal/redox mod- ulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability. The Journal of Physiology, Wiley, 2016, 594 (13), pp.3561–74. 10.1113/JP271925. hal-01940961 HAL Id: hal-01940961 https://hal.archives-ouvertes.fr/hal-01940961 Submitted on 30 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J Physiol 594.13 (2016) pp 3561–3574 3561 Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin1,2,3, Emmanuel Bourinet1,2,3 and Philippe Lory1,2,3 1Centre National pour la Recherche Scientifique UMR 5203, D´epartement de Physiologie, Institut de G´enomique Fonctionnelle, Universit´edeMontpellier, Montpellier, F-34094 France 2Institut National de la Sant´eetdelaRechercheM´edicale, U 1191, Montpellier, F-34094 France Neuroscience 3LabEx ‘Ion Channel Science and Therapeutics’, Montpellier, F-34094 France Key points r In this study, we describe a new knock-in (KI) mouse model that allows the study of the H191-dependent regulation of T-type Cav3.2 channels. -

Hyperkalemia Masked by Pseudo-Stemi Infarct Pattern and Cardiac Arrest Shareez Peerbhai1 , Luke Masha2, Adrian Dasilva-Deabreu1 and Abhijeet Dhoble1,2*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Peerbhai et al. International Journal of Emergency Medicine (2017) 10:3 International Journal of DOI 10.1186/s12245-017-0132-0 Emergency Medicine CASEREPORT Open Access Hyperkalemia masked by pseudo-stemi infarct pattern and cardiac arrest Shareez Peerbhai1 , Luke Masha2, Adrian DaSilva-DeAbreu1 and Abhijeet Dhoble1,2* Abstract Background: Hyperkalemia is a common electrolyte abnormality and has well-recognized early electrocardiographic manifestations including PR prolongation and symmetric T wave peaking. With severe increase in serum potassium, dysrhythmias and atrioventricular and bundle branch blocks can be seen on electrocardiogram. Although cardiac arrest is a worrisome consequence of untreated hyperkalemia, rarely does hyperkalemia electrocardiographically manifest as acute ischemia. Case presentation: We present a case of acute renal failure complicated by malignant hyperkalemia and eventual ventricular fibrillation cardiac arrest. Recognition of this disorder was delayed secondary to an initial ECG pattern suggesting an acute ST segment elevation myocardial infarction (STEMI). Emergent coronary angiography performed showed no evidence of coronary artery disease. Conclusions: Pseudo-STEMI patterns are rarely seen in association with acute hyperkalemia and are most commonly described with patient without acute cardiac symptomatology. This is the first such case presenting concurrently with cardiac arrest. A brief review of this rare pseudo-infarct pattern is also given. Keywords: Cardiac arrest, Hyperkalemia, Myocardial infarction, STEMI, ECG Background Case presentation Hyperkalemia that manifests with electrocardiographic A 27-year-old Caucasian male with a past medical his- findings of an ST segment elevation myocardial infarc- tory of hypertension presented to the emergency room tion (STEMI) is very rare. -

Increased Mortality Associated with Hypermagnesemia in Severe COVID-19 Illness
Original Investigation Increased Mortality Associated with Hypermagnesemia in Severe COVID-19 Illness Jacob S. Stevens 1,2, Andrew A. Moses1, Thomas L. Nickolas1,2, Syed Ali Husain1,2, and Sumit Mohan1,2,3 Key Points Hypermagnesemia is common in patients admitted with coronavirus disease 2019. The development of hypermagnesemia in coronavirus disease 2019 is associated with renal failure and markers of high cell turnover. In adjusted models, patients who develop hypermagnesemia have an increased risk of mortality. Abstract Background Although electrolyte abnormalities are common among patients with COVID-19, very little has been reported on magnesium homeostasis in these patients. Here we report the incidence of hypermagnesemia, and its association with outcomes among patients admitted with COVID-19. Methods We retrospectively identified all patients with a positive test result for SARS-CoV-2who were admitted to a large quaternary care center in New York City in spring 2020. Details of the patients’ demographics and hospital course were obtained retrospectively from medical records. Patients were defined as having hypermagnesemia if their median magnesium over the course of their hospitalization was .2.4 mg/dl. Results A total of 1685 patients hospitalized with COVID-19 had their magnesium levels checked during their hospitalization, and were included in the final study cohort, among whom 355 (21%) had hypermagnesemia. Patients who were hypermagnesemic had a higher incidence of shock requiring pressors (35% vs 27%, P,0.01), respiratory failure requiring mechanical ventilation (28% vs 21%, P50.01), AKI (65% vs 50%, P,0.001), and AKI severe enough to require renal replacement therapy (18% vs 5%, P,0.001). -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

Early-Onset Neonatal Hyperkalemia Associated with Maternal
Tanaka et al. BMC Pediatrics (2018) 18:55 https://doi.org/10.1186/s12887-018-1048-4 CASEREPORT Open Access Early-onset neonatal hyperkalemia associated with maternal hypermagnesemia: a case report Kenichi Tanaka1, Hiroko Mori1, Rieko Sakamoto2, Shirou Matsumoto2, Hiroshi Mitsubuchi1, Kimitoshi Nakamura2 and Masanori Iwai1* Abstract Background: Neonatal nonoliguric hyperkalemia (NOHK) is a metabolic abnormality that occurs in extremely premature neonates at approximately 24 h after birth and is mainly due to the immature functioning of the sodium (Na+)/potassium (K+) pump. Magnesium sulfate is frequently used in obstetrical practice to prevent preterm labor and to treat preeclampsia; this medication can also cause hypermagnesemia and hyperkalemia by a mechanism that is different from that of NOHK. Herein, we report the first case of very early-onset neonatal hyperkalemia induced by maternal hypermagnesemia. Case presentation: A neonate born at 32 weeks of gestation developed hyperkalemia (K+ 6.4 mmol/L) 2 h after birth. The neonate’s blood potassium concentration reached 7.0 mmol/L 4 h after birth, despite good urine output. The neonate and his mother had severe hypermagnesemia caused by intravenous infusion of magnesium sulfate given for tocolysis due to pre-term labor. Conclusion: The early-onset hyperkalemia may have been caused by the accumulation of potassium ions transported through the placenta, the shift of potassium ions from the intracellular to the extracellular space in the infant due to the malfunctioning of the Na+/K+ pump and the inhibition of renal distal tube potassium ion secretion, there is a possibility that these mechanisms were induced by maternal and fetal hypermagnesemia after maternal magnesium sulfate administration. -
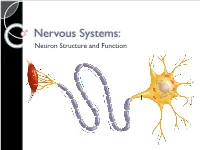
4-Nervous-System-Structure-PPT.Pdf
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation and coordination that produce coherency and result in harmonious function. Integration Cellular integration = processes within cells Whole-animal integration = selective combination and processing of sensory, endocrine, and central nervous system (CNS) information in ways that promote harmonious functioning of the whole organism within its environment. ◦ This includes its all its cells, tissues, and organs Integration Nerve cells are specialized for control and coordination. Integration ensures that an animal’s responses are smooth and coordinated rather than clashing or disjointed. Excitable Cells Neurons are a type of excitable cell ◦ Specially adapted to generate an electrical signal Can rapidly alter their membrane potential in response to an incoming signal. Vary in structure and function but use the same basic mechanism to send signals. Neuron Function – Main Points Specialized for processing and conveying information. Information is coded into changes in electrical potential across cell membranes. Neurons use action potentials to transmit these signals across long distances. Neurons allow animals to sense and respond to their environment. Benefits of Neurons Plants (no neurons): Action potentials travel @ 1-3 cm/sec Animals (neurons): Action potentials travel @ 100m/sec or 10,000cm/sec CNS to Muscles Signal Reception Dendrites & Cell Body Signal Integration Axon Hillock Signal Conduction Axon Signal Transmission Axon Terminals Signal Reception Dendrites sense and convert incoming signals into electrical signals by changing membrane potential. Cell Body = routine metabolic functions Signal Integration Incoming signals are conducted to the axon hillock If signal is sufficiently large an electrical signal, or action potential, is initiated. -

Two Components of the Cardiac Action Potential I
Two Components of the Cardiac Action Potential I. Voltage-time course and the effect of acet.flcholine on atrial and nodal cells of the rabbit heart ANTONIO PAES de CARVALHO, BRIAN FRANCIS HOFFMAN, and MARILENE de PAULA CARVALHO From the Instituto de Biofisica da Universidade Federal do Rio de Janeiro, Rio de Janeiro (GB), Brazil, and the Department of Pharmacology, College of Physicians and Surgeons, Columbia University, New York 10032 ABSTRACT Transmembrane potentials recorded from the rabbit heart in vitro were displayed as voltage against time (V, t display), and dV/dt against voltage (I?, V or phase-plane display). Acetylcholine was applied to the record- ing site by means of a hydraulic system. Results showed that (a) differences in time course of action potential upstroke can be explained in terms of the rela- tive magnitude of fast and slow phases of depolarization; (b) acetylcholine is capable of depressing the slow phase of depolarization as well as the plateau of the action potential; and (¢) action potentials from nodal (SA and AV) cells seem to lack the initial fast phase. These results were construed to support a two-component hypothesis for cardiac electrogenesis. The hypothesis states that cardiac action potentials are composed of two distinct and physiologically separable "components" which result from discrete mechanisms. An initial fast component is a sodium spike similar to that of squid nerve. The slow com- ponent, which accounts for both a slow depolarization during phase 0 and the plateau, probably is dependent on the properties of a slow inward current hav- ing a positive equilibrium potential, coupled to a decrease in the resting potas- sium conductance. -

Front Matter (PDF)
HIGH RATE OF SUCCESS IN AN NIH- STUDY of hypertensive patients- highest percentage - remained on itial therapy 83% with NORVASC#{174}(amlodipi besylate) after 4 years; nearly all pati were on the 5-mg starting dose’ LOW RATE DISCONTINUATION ONLY 1.5%of patients in placebo studies (n= 1730) discontinued due to adverse effects2 PROVEN No negative inotropic effects clinical doses in hemodyna m ic stud 2* No clinically significant effect on cardiac conduction or heart rate2 *S1IflH,jr fiernodynamic findings, hcvever fiave been agents possessing significant neqafive notropic ebecto and 10-mg tablets Once-Daily NORV (ambdipine EFFICACY AND SAFETY THAT’S EASY TO WITH Br$e(Summary NORVASC(amlodlpine besylate) Tablets For Oral Use CONTRAINDICATIONS: NORVASC is contraindicated in patients with known sensitivity to arniodipirre. In hypertension WARNINGS: Increased Angina and/or MyOcardIal Infarction: Rarely, patients, particulady those with severe obstruc5ve coronary artery disease, have developed documented increased frequency,durationand/or severity of angina or acute myocardial infarction on starhng cafrium channel blocker therapy or attire time of dosage increase. The mechanism of this effect has not been elucidated. PRECAUTIONS: General: Since the vasoditation induced by NORVASC is gradual in onset, acute hypotension has rarely been reported after oraladministrationof NORVASC. Nonethetuss, caution should be exercised when admin- or angina, convenient istering NORVASC as with any other penpherio vasodilator particularly in pahentswithsevere