Two Components of the Cardiac Action Potential I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Rate Information Sheet Finding Your Pulse Taking Your Pulse
Heart rate In this activity you will measure your heart rate and investigate the effect that other things have on your heart rate. Information sheet You can measure your heart (pulse) rate anywhere on your body where a major artery is close to the surface of your skin. The easiest places are: • on the front of your forearm • just above your wrist on your thumb side • on the side of your neck about half way between your chin and your ear. Finding your pulse Try to locate your pulse in one of these places, using the tips of your index and middle fingers. You should feel a gentle, regular beat. This is your heart rate. Do not use your thumb, as your thumb has a pulse of its own. Taking your pulse When you find your pulse, use a stopwatch or a watch with a second hand to count how many beats there are in a full minute (60 seconds). In most situations, taking your pulse rate over one minute will give a reasonably accurate result, but if you want to take your pulse after exercise, you should do so over a much shorter time interval. After exercise your pulse rate will be changing rapidly. To get a reasonably accurate result, start to measure the rate immediately after the exercise and count the number of beats in 10 seconds. Multiply this number by six to find your heart rate. For an adult, a normal resting heart rate is between 60–100 beats a minute. The fitter you are, the lower your resting heart beat will be. -

Heart to Heart - STEAM Activity
Heart to Heart - STEAM Activity Purpose: The main objective of this exercise is to introduce how the human heart works. This lesson will be divided into four different lessons, including an introduction to heart anatomy, heart beats and pulses, and the circulatory system. We will be using coloring activities, stethoscopes, and handmade pumps to reinforce the concepts seen in this lesson. Vocabulary ● Artery: A blood vessel that carries blood high in oxygen content away from the heart to the farthest reaches of the body. ● Vein: a Blood vessel that carries blood low in oxygen content from the body back to the heart. ● Atrium: One of the two upper cavities of the heart that passes blood to the ventricles. ● Ventricles: One of the two lower chambers of the heart that receives blood from the atria. ● Valves: Tissue-paper thin membranes attached to the heart wall that constantly open and close to regulate blood flow. ● Pulse: A rhythmical, mechanical throbbing of the arteries as blood pumps through them. ● Heart Rate: The number of times per minute that the heart contracts - the number of heart beats per minute (bpm). ● Taquicardia: A high resting heart rate that is usually higher than 100 beats per minute. ● Bradycardia: A low resting heart rate that is usually lower than 60 beats per minute. ● Circulation: The movement of blood through the vessels of the body by the pumping action of the heart. It distributes nutrients and oxygen and removes waste products from all parts of the body. ● Pulmonary Circulation: The portion of the circulatory system that carries deoxygenated blood from the heart to the lungs and oxygenated blood back to the heart. -

Toolbox-Talks--Blood-Pressure.Pdf
TOOLBOX Toolbox Talk #1 TALKS Blood Pressure vs. Heart Rate While your blood pressure is the force of your blood moving through your blood vessels, your heart rate is the number of times your heart beats per minute. They are two separate measurements and indicators of health. • For people with high blood pressure (HBP or hypertension), there’s no substitute for measuring blood pressure. • Heart rate and blood pressure do not necessarily increase at the same rate. A rising heart rate does not cause your blood pressure to increase at the same Quarter: rate. Even though your heart is beating more times a minute, healthy blood BLOOD vessels dilate (get larger) to allow more blood to flow through more easily. PRESSURE When you exercise, your heart speeds up so more blood can reach your muscles. It may be possible for your heart rate to double safely, while your blood pressure may respond by only increasing a modest amount. Talk Number: Heart Rate and Exercise 1 In discussions about high blood pressure, you will often see heart rate Blood mentioned in relation to exercise. Your target heart rate is based on age and Pressure can help you monitor the intensity of your exercise. vs. • If you measure your heart rate (take your pulse) before, during and after Heart Rate physical activity, you’ll notice it will increase over the course of the exercise. • The greater the intensity of the exercise, the more your heart rate will increase. • When you stop exercising, your heart rate does not immediately return to your normal (resting) heart rate. -

Cardiovascular Physiology
CARDIOVASCULAR PHYSIOLOGY Ida Sletteng Karlsen • Trym Reiberg Second Edition 15 March 2020 Copyright StudyAid 2020 Authors Trym Reiberg Ida Sletteng Karlsen Illustrators Nora Charlotte Sønstebø Ida Marie Lisle Amalie Misund Ida Sletteng Karlsen Trym Reiberg Booklet Disclaimer All rights reserved. No part oF this book may be reproduced in any Form on by an electronic or mechanical means, without permission From StudyAid. Although the authors have made every efFort to ensure the inFormation in the booklet was correct at date of publishing, the authors do not assume and hereby disclaim any liability to any part For any inFormation that is omitted or possible errors. The material is taken From a variety of academic sources as well as physiology lecturers, but are Further incorporated and summarized in an original manner. It is important to note, the material has not been approved by professors of physiology. All illustrations in the booklet are original. This booklet is made especially For students at the Jagiellonian University in Krakow by tutors in the StudyAid group (students at JU). It is available as a PDF and is available for printing. If you have any questions concerning copyrights oF the booklet please contact [email protected]. About StudyAid StudyAid is a student organization at the Jagiellonian University in Krakow. Throughout the academic year we host seminars in the major theoretical subjects: anatomy, physiology, biochemistry, immunology, pathophysiology, supplementing the lectures provided by the university. We are a group of 25 tutors, who are students at JU, each with their own Field oF specialty. To make our seminars as useful and relevant as possible, we teach in an interactive manner often using drawings and diagrams to help students remember the concepts. -

7.016 Introductory Biology Fall 2018
7.016: Fall 2018: MIT 7.016 Recitation 15 – Fall 2018 (Note: The recitation summary should NOT be regarded as the substitute for lectures) (This material is COPYRIGHT protected.) Summary of Lecture 22 (11/2): Neurons and action potentials: Ions can move across membranes through pumps and channels. Integral membrane proteins like these cross the membrane via transmembrane domains. Pumps are ATPases that set up the concentration gradients of ions across cell membranes, such that K+ is high inside cells and other ions (such as Cl–, Na+, and Ca2+) are high outside cells. A membrane potential is only set up by ions that move freely across the membrane through open channels, creating one side of the membrane that is more positive relative to the other side. This movement of ions does not dissipate the concentration gradient because the number of ions that move to generate a membrane potential is very small compared to the number of ions that need to be pumped to create a concentration gradient. Most cell membranes only contain open K+ channels and thus only K+ flows freely across membranes through channels; K+ is high inside, causing it to flow outside, giving the inside of cells a negative membrane potential. Ions can move across the membrane through open ion channels. Two forces act to dictate this movement – the concentration gradient and the electrical gradient. Ions move down their concentration gradient through channels, and ions move towards the side of the membrane that harbors the opposite charge. Neurons are the cells of your nervous system that make connections with each other to transmit impulse/ signals. -

Non-Coding Rnas in the Cardiac Action Potential and Their Impact on Arrhythmogenic Cardiac Diseases
Review Non-Coding RNAs in the Cardiac Action Potential and Their Impact on Arrhythmogenic Cardiac Diseases Estefania Lozano-Velasco 1,2 , Amelia Aranega 1,2 and Diego Franco 1,2,* 1 Cardiovascular Development Group, Department of Experimental Biology, University of Jaén, 23071 Jaén, Spain; [email protected] (E.L.-V.); [email protected] (A.A.) 2 Fundación Medina, 18016 Granada, Spain * Correspondence: [email protected] Abstract: Cardiac arrhythmias are prevalent among humans across all age ranges, affecting millions of people worldwide. While cardiac arrhythmias vary widely in their clinical presentation, they possess shared complex electrophysiologic properties at cellular level that have not been fully studied. Over the last decade, our current understanding of the functional roles of non-coding RNAs have progressively increased. microRNAs represent the most studied type of small ncRNAs and it has been demonstrated that miRNAs play essential roles in multiple biological contexts, including normal development and diseases. In this review, we provide a comprehensive analysis of the functional contribution of non-coding RNAs, primarily microRNAs, to the normal configuration of the cardiac action potential, as well as their association to distinct types of arrhythmogenic cardiac diseases. Keywords: cardiac arrhythmia; microRNAs; lncRNAs; cardiac action potential Citation: Lozano-Velasco, E.; Aranega, A.; Franco, D. Non-Coding RNAs in the Cardiac Action Potential 1. The Electrical Components of the Adult Heart and Their Impact on Arrhythmogenic The adult heart is a four-chambered organ that propels oxygenated blood to the entire Cardiac Diseases. Hearts 2021, 2, body. It is composed of atrial and ventricular chambers, each of them with distinct left and 307–330. -

Genetic Alteration of the Metal/Redox Modulation of Cav3.2 T-Type
Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory To cite this version: Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory. Genetic alteration of the metal/redox mod- ulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability. The Journal of Physiology, Wiley, 2016, 594 (13), pp.3561–74. 10.1113/JP271925. hal-01940961 HAL Id: hal-01940961 https://hal.archives-ouvertes.fr/hal-01940961 Submitted on 30 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J Physiol 594.13 (2016) pp 3561–3574 3561 Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin1,2,3, Emmanuel Bourinet1,2,3 and Philippe Lory1,2,3 1Centre National pour la Recherche Scientifique UMR 5203, D´epartement de Physiologie, Institut de G´enomique Fonctionnelle, Universit´edeMontpellier, Montpellier, F-34094 France 2Institut National de la Sant´eetdelaRechercheM´edicale, U 1191, Montpellier, F-34094 France Neuroscience 3LabEx ‘Ion Channel Science and Therapeutics’, Montpellier, F-34094 France Key points r In this study, we describe a new knock-in (KI) mouse model that allows the study of the H191-dependent regulation of T-type Cav3.2 channels. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

Jugular Venous Pressure
NURSING Jugular Venous Pressure: Measuring PRACTICE & SKILL What is Measuring Jugular Venous Pressure? Measuring jugular venous pressure (JVP) is a noninvasive physical examination technique used to indirectly measure central venous pressure(i.e., the pressure of the blood in the superior and inferior vena cava close to the right atrium). It is a part of a complete cardiovascular assessment. (For more information on cardiovascular assessment in adults, see Nursing Practice & Skill ... Physical Assessment: Performing a Cardiovascular Assessment in Adults ) › What: Measuring JVP is a screening mechanism to identify abnormalities in venous return, blood volume, and right heart hemodynamics › How: JVP is determined by measuring the vertical distance between the sternal angle and the highest point of the visible venous pulsation in the internal jugular vein orthe height of the column of blood in the external jugular vein › Where: JVP can be measured in inpatient, outpatient, and residential settings › Who: Nurses, nurse practitioners, physician assistants, and treating clinicians can measure JVP as part of a complete cardiovascular assessment What is the Desired Outcome of Measuring Jugular Venous Pressure? › The desired outcome of measuring JVP is to establish the patient’s JVP within the normal range or for abnormal JVP to be identified so that appropriate treatment may be initiated. Patients’ level of activity should not be affected by having had the JVP measured ICD-9 Why is Measuring Jugular Venous Pressure Important? 89.62 › The JVP is -
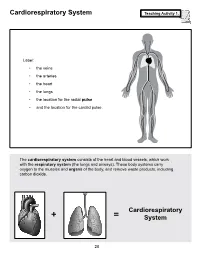
Cardiorespiratory System Teaching Activity 1
Cardiorespiratory System Teaching Activity 1. Label: • the veins • the arteries • the heart • the lungs • the location for the radial pulse • and the location for the carotid pulse. The cardiorespiratory system consists of the heart and blood vessels, which work with the respiratory system (the lungs and airways). These body systems carry oxygen to the muscles and organs of the body, and remove waste products, including carbon dioxide. Cardiorespiratory + = System 28 The Most Important Muscle: The Heart Teaching Activity 1, continued. In the circulatory system, the heart is a muscle that acts as a pump. In fact, the heart is a double pump. Blood that needs oxygen enters the heart and is pumped by the first pump to the lungs. The second pump of the heart pumps the oxygen-rich blood to all the other parts of the body. This gives the heart its common, “lub-dub” sound. The number of times the heart pumps, or beats, is counted in a minute. This is known as the pulse rate. A person’s pulse is affected by their current level of activity. If you are sleeping or do no physical activity at all, your heart is pumping at a resting heart rate. When active, you are using all your body systems. These systems require fuel in the form of calories (found in food), and oxygen (what you breathe). The more active you are, the Hearty Facts more fuel your muscles need. This is why your breathing rate and your heart rate increase when • Your system of blood vessels (arteries, you exercise. veins, and capillaries) is over 60,000 miles long. -

Cardiovascular System: Heart
Cardiovascular System: Heart Cardiovascular System – Heart Conducting cells: Cardiac Electrophysiology Cardiac cells specialized to quickly spread action potentials across myocardium • Weak force generators System allows for orderly, sequential depolarization and Intrinsic Conduction System: contraction of heart Normal sinus rhythm: 1) AP originates at SA node 2) SA node fires at 60 – 100 beats / min Atrial internodal tracts Sinoatrial node: (SA node) 3) Correct myocardial activation sequence • Located in right atrial wall • Initiates action potentials (APs) • Pacemaker (~ 80 beats / min) Bundle branches Atrioventricular node: (AV Node) • Connects atria to ventricles • Slowed conduction velocity • Ventricular filling Purkinje Bundle of His fibers Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 18.14 1 Cardiovascular System – Heart Cardiac Electrophysiology The autonomic nervous system can directly affect the heart rate; these effects are called chronotropic effects Recall: spontaneous depolarization = VG Na+ channels Positive chronotrophic effects: (increase heart rate) • Under sympathetic control Leads to g ; cells SA Na NE node reach threshold more rapidly Sinoatrial node Pharmacology: β1 receptors β-blockers (e.g., propanolol) Negative chronotrophic effects: (decrease heart rate) • Under parasympathetic control Leads to gNa; cells reach threshold SA less rapidly ACh node Leads to gK; cells hyperpolarized during Muscarinic receptors repolarization stage (further from threshold) Costanzo (Physiology, 4th ed.) – Figure