Neuropathic Pain Therapeutic Class Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Preventive Report Appendix
Title Authors Published Journal Volume Issue Pages DOI Final Status Exclusion Reason Nasal sumatriptan is effective in treatment of migraine attacks in children: A Ahonen K.; Hamalainen ML.; Rantala H.; 2004 Neurology 62 6 883-7 10.1212/01.wnl.0000115105.05966.a7 Deemed irrelevant in initial screening Seasonal variation in migraine. Alstadhaug KB.; Salvesen R.; Bekkelund SI. Cephalalgia : an 2005 international journal 25 10 811-6 10.1111/j.1468-2982.2005.01018.x Deemed irrelevant in initial screening Flunarizine, a calcium channel blocker: a new prophylactic drug in migraine. Amery WK. 1983 Headache 23 2 70-4 10.1111/j.1526-4610.1983.hed2302070 Deemed irrelevant in initial screening Monoamine oxidase inhibitors in the control of migraine. Anthony M.; Lance JW. Proceedings of the 1970 Australian 7 45-7 Deemed irrelevant in initial screening Prostaglandins and prostaglandin receptor antagonism in migraine. Antonova M. 2013 Danish medical 60 5 B4635 Deemed irrelevant in initial screening Divalproex extended-release in adolescent migraine prophylaxis: results of a Apostol G.; Cady RK.; Laforet GA.; Robieson randomized, double-blind, placebo-controlled study. WZ.; Olson E.; Abi-Saab WM.; Saltarelli M. 2008 Headache 48 7 1012-25 10.1111/j.1526-4610.2008.01081.x Deemed irrelevant in initial screening Divalproex sodium extended-release for the prophylaxis of migraine headache in Apostol G.; Lewis DW.; Laforet GA.; adolescents: results of a stand-alone, long-term open-label safety study. Robieson WZ.; Fugate JM.; Abi-Saab WM.; 2009 Headache 49 1 45-53 10.1111/j.1526-4610.2008.01279.x Deemed irrelevant in initial screening Safety and tolerability of divalproex sodium extended-release in the prophylaxis of Apostol G.; Pakalnis A.; Laforet GA.; migraine headaches: results of an open-label extension trial in adolescents. -

Topical Therapy As a Treatmentfor Brachioradial
Journal of Case Reports: Open Access Case report Open Access Topical Therapy as a Treatmentfor Brachioradial Pruritis: a Case Report Brianna De Souza M.D, Amy McMichael M.D* Department of Dermatology, Wake Forest University School of Medicine, Winston-Salem, North Carolina *Corresponding author: Amy McMichael, MD Department of Dermatology, Wake Forest Baptist Medical Center,1 Medical Center Blvd, Winston-Salem, NC 27157, Phone: 336-716-7882, Email: [email protected] Received Date: April 12, 2019 Accepted Date: May 06, 2019 Published Date: May 08, 2019 Citation: Brianna De Souza (2019) Topical Therapy as a Treatmentfor Brachioradial Pruritis: a Case Report. Case Reports: Open Access 4: 1-5. Abstract Management of brachioradial pruritus (BRP) presents a formidable challenge to dermatologists and neurologists. BRP is a rare, neurocutaneous condition characterized by sharply localized, chronic pain with associated itching, burning, stinging, and or tingling sensation. Effective care of this patient population is confounded by limitations within the litera- ture, comprised of case series and case reports. We present a case of one middle-aged female with a chronic history of BRP recalcitrant to the following oral therapies: pregabalin, gabapentin, mirtazapine, prednisone, and amitriptyline, as well as topical triamcinolone. After being evaluated in the clinic, the patient was started on combination therapy withKetamine 10%, Amitriptyline 5%, and Lidocaine 5% topical cream to which she responded. Keywords: Brachioradial pruritus, Brachioradial, Pruritus, Neurocutaneous ©2019 The Authors. Published by the JScholar under the terms of the Crea- tive Commons Attribution License http://creativecommons.org/licenses/ by/3.0/, which permits unrestricted use, provided the original author and source are credited. -

Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs
ISSN 2380-5064 | The Arsenal is published by the Augusta University Libraries | http://guides.augusta.edu/arsenal Volume 4, Issue 1 (2021) Special Edition Issue CONJUGATION OF TRYPTAMINE TO BIOLOGICALLY ACTIVE CARBOXYLIC ACIDS IN ORDER TO CREATE PRODRUGS Colin Miller, Jr. and Iryna O. Lebedyeva Citation Miller, C., Jr., & Lebedyeva, I. O. (2021). Conjugation of tryptamine to biologically active carboxylic acids in order to create prodrugs. The Arsenal: The Undergraduate Research Journal of Augusta University, 4(1), 26. http://doi.org/10.21633/issn.2380.5064/s.2021.04.01.26 © Miller, Jr. and Lebedyeva 2021. This open access article is distributed under a Creative Commons Attribution NonCommercial-NoDerivs 2.0 Generic License (https://creativecommons.org/licenses/by-nc-nd/2.0/). Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs Presenter(s): Colin Miller, Jr. Author(s): Colin Miller Jr. and Iryna O. Lebedyeva Faculty Sponsor(s): Iryna O. Lebedyeva, PhD Affiliation(s): Department of Chemistry and Physics (Augusta Univ.) ABSTRACT A number of blood and brain barrier penetrating neurotransmitters contain polar functional groups. Gamma-aminobutyric acid (GABA) is an amino acid, which is one of the primary inhibitory neurotransmitter in the brain and a major inhibitory neurotransmitter in the spinal cord. Antiepileptic medications such as Gabapentin, Phenibut, and Pregabalin have been developed to structurally represent GABA. These drugs are usually prescribed for the treatment of neuropathic pain. Since these drugs contain a polar carboxylic acid group, it affects their ability to penetrate the blood and brain barrier. To address the low bioavailability and tendency for intramolecular cyclization of Gabapentin, its less polar prodrug Gabapentin Enacarbil has been approved in 2011. -

(12) United States Patent (10) Patent No.: US 8,283,487 B2 Ben Moha-Lerman Et Al
USOO8283487B2 (12) United States Patent (10) Patent No.: US 8,283,487 B2 Ben Moha-Lerman et al. (45) Date of Patent: Oct. 9, 2012 (54) PROCESSES FOR THE PREPARATION AND 2003/0176398 A1 9/2003 Gallop et al. PURIFICATION OF GABAPENTIN 2004/0077553 A1* 4/2004 Gallop et al. ................... 514, 19 2005/O154057 A1 7/2005 Estrada et al. ENACARBL 2007/0049627 A1 3, 2007 Tran (75) Inventors: Elena Ben Moha-Lerman, Kiryat Ono FOREIGN PATENT DOCUMENTS (IL); Tamar Nidam, Yehud (IL); Meital WO WO O2/10O347 12/2002 Cohen, Petach-Tikva (IL); Sharon WO WO 2005/0377.84 4/2005 Avhar-Maydan, Givataym (IL); Anna Balanov, Rehovot (IL) OTHER PUBLICATIONS X. Yuan et al. “In Situ Preparation of Zinc Salts of Unsaturated (73) Assignee: Teva Pharmaceutical Industries Ltd., Carboxylic Acids to Reinforce NBR' J. Applied Polymer Science, Petach-Tikva (IL) vol. 77, p. 2740-2748 (2000). - r J. Alexander et al. “(Acyloxy)alkyl Carbamates as Novel Biorevers (*) Notice: Subject to any disclaimer, the term of this ible Prodrugs for Amines: Increased Permeation through Biological patent is extended or adjusted under 35 Membranes”.J. Med. Chem. 1988, 31, pp. 318-322. U.S.C. 154(b) by 249 days. S.M. Rahmathullah et al. “Prodrugs for Amidines: Synthesis and Anti-Pneumocystis carinii Activity of Carbamates of 2.5-Bis(4- (21) Appl. No.: 12/626,682 amidinophenyl)furan” J. Med. Chem. 1999, 42, pp. 3994-4000. (22) Filed: Nov. 26, 2009 * cited by examiner (65) Prior Publication Data Primary Examiner — Joseph K. McKane US 2010/O160665 A1 Jun. 24, 2010 Assistant Examiner — Alicia L Otton (74) Attorney, Agent, or Firm — Arent Fox LLP Related U.S. -

More Treatments on Deck for Alcohol Use Disorder
News & Analysis Medical News & Perspectives More Treatments on Deck for Alcohol Use Disorder Jeff Lyon hirteen years have passed since the Raye Z. Litten, PhD, acting director of the ment for AUD were actually prescribed an US Food and Drug Administration NIAAA’s Division of Medications Develop- AUD medication. T (FDA) last approved a new medica- ment and principal investigator of the trial, It is a complicated problem, says John tion to help the nation’s millions of people says the agency is “excited.” Rotrosen, MD, a psychiatrist and addiction withalcoholusedisorder(AUD)stopormod- Nevertheless, the science of alcohol ad- specialist at New York University School of erate their drinking. diction has seen its share of medicines that Medicine. “To a large extent primary care Only 3 such formulations exist, and 1, failed to live up to their early promise dur- physicians don’t screen for alcoholism,” he disulfiram, dates to the Prohibition era. ing full-scale testing. So Litten said the said. “When they do, they may note it, but Known commercially as Antabuse and agency isn’t putting all its eggs in one bas- don’t really do anything about it.” introduced in 1923, it makes people memo- ket. “You need as many weapons as pos- As far as the failure to prescribe exist- rably ill if they ingest alcohol, but it doesn’t sible to treat a complex disease like alcohol ing medications for AUD, Rotrosen blames stop the cravings. The other 2, naltrexone use disorder,” he said. a lack of knowledge. “A lot of doctors in and acamprosate, approved in 1994 and But therein lies a second difficulty. -

Antagonism of Lidocaine Inhibition by Open-Channel Blockers That Generate Resurgent Na Current
4976 • The Journal of Neuroscience, March 13, 2013 • 33(11):4976–4987 Cellular/Molecular Antagonism of Lidocaine Inhibition by Open-Channel Blockers That Generate Resurgent Na Current Jason S. Bant,1,3 Teresa K. Aman,2,3 and Indira M. Raman1,2,3 1Interdepartmental Biological Sciences Program, 2Northwestern University Interdepartmental Neuroscience Program, and 3Department of Neurobiology, Northwestern University, Evanston, Illinois 60208 Na channels that generate resurgent current express an intracellular endogenous open-channel blocking protein, whose rapid binding upon depolarization and unbinding upon repolarization minimizes fast and slow inactivation. Na channels also bind exogenous com- pounds, such as lidocaine, which functionally stabilize inactivation. Like the endogenous blocking protein, these use-dependent inhibi- tors bind most effectively at depolarized potentials, raising the question of how lidocaine-like compounds affect neurons with resurgent Na current. We therefore recorded lidocaine inhibition of voltage-clamped, tetrodotoxin-sensitive Na currents in mouse Purkinje neu- rons, which express a native blocking protein, and in mouse hippocampal CA3 pyramidal neurons with and without a peptide from the   cytoplasmic tail of NaV 4 (the 4 peptide), which mimics endogenous open-channel block. To control channel states during drug exposure, lidocaine was applied with rapid-solution exchange techniques during steps to specific voltages. Inhibition of Na currents by lidocaine was diminished by either the 4 peptide or the native blocking protein. In peptide-free CA3 cells, prolonging channel opening with a site-3 toxin, anemone toxin II, reduced lidocaine inhibition; this effect was largely occluded by open-channel blockers, suggesting that lidocaine binding is favored by inactivation but prevented by open-channel block. -

Diabetic Neuropathy: a Position Statement by the American
136 Diabetes Care Volume 40, January 2017 Diabetic Neuropathy: A Position Rodica Pop-Busui,1 Andrew J.M. Boulton,2 Eva L. Feldman,3 Vera Bril,4 Roy Freeman,5 Statement by the American Rayaz A. Malik,6 Jay M. Sosenko,7 and Dan Ziegler8 Diabetes Association Diabetes Care 2017;40:136–154 | DOI: 10.2337/dc16-2042 Diabetic neuropathies are the most prevalent chronic complications of diabetes. This heterogeneous group of conditions affects different parts of the nervous system and presents with diverse clinical manifestations. The early recognition and appropriate man- agement of neuropathy in the patient with diabetes is important for a number of reasons: 1. Diabetic neuropathy is a diagnosis of exclusion. Nondiabetic neuropathies may be present in patients with diabetes and may be treatable by specific measures. 2. A number of treatment options exist for symptomatic diabetic neuropathy. 3. Up to 50% of diabetic peripheral neuropathies may be asymptomatic. If not recognized and if preventive foot care is not implemented, patients are at risk for injuries to their insensate feet. 4. Recognition and treatment of autonomic neuropathy may improve symptoms, POSITION STATEMENT reduce sequelae, and improve quality of life. Among the various forms of diabetic neuropathy, distal symmetric polyneurop- athy (DSPN) and diabetic autonomic neuropathies, particularly cardiovascular au- tonomic neuropathy (CAN), are by far the most studied (1–4). There are several 1 atypical forms of diabetic neuropathy as well (1–4). Patients with prediabetes may Division of Metabolism, Endocrinology & Diabe- – tes, Department of Internal Medicine, University also develop neuropathies that are similar to diabetic neuropathies (5 10). -
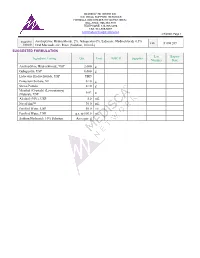
Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 Ml)
MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 4/7/2020; Page 1 Suggested Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 mL) SUGGESTED FORMULATION Lot Expiry Ingredient Listing Qty. Unit NDC # Supplier Number Date Amitriptyline Hydrochloride, USP 2.000 g Gabapentin, USP 6.000 g Lidocaine Hydrochloride, USP TBD Potassium Sorbate, NF 0.10 g Stevia Powder 0.10 g Menthol (Crystals) (Levorotatory) 0.02 g (Natural), USP Alcohol (95%), USP 5.0 mL NovaFilm™ 30.0 mL Purified Water, USP 50.0 mL Purified Water, USP q.s. to 100.0 mL Sodium Hydroxide 10% Solution As required MEDISCA® NETWORK INC. TECHNICAL SUPPORT SERVICES FORMULATION CHEMISTRY DEPARTMENT TOLL-FREE: 866-333-7811 TELEPHONE: 514-905-5096 FAX: 514-905-5097 [email protected] 4/7/2020; Page 2 Suggested Amitriptyline Hydrochloride 2%, Gabapentin 6%, Lidocaine Hydrochloride 0.5% FIN F 008 269 Formula Oral Mucoadhesive Rinse (Solution, 100 mL) SPECIAL PREPARATORY CONSIDERATIONS Ingredient-Specific Information Light Sensitive (protect from light whenever possible): Amitriptyline Hydrochloride, Gabapentin Hygroscopic (protect from moisture whenever possible): Stevia Powder Narrow Therapeutic Index Lidocaine Hydrochloride Suggested Preparatory Guidelines ■ Non-Sterile Preparation □ Sterile Preparation Processing Error / To account for processing error and pH testing considerations during preparation, it is Testing Considerations: suggested to measure an additional 3 to 5% of the required quantities of ingredients. Special Instruction: This formula may contain one or more Active Pharmaceutical Ingredients (APIs) that may be classified as hazardous, please refer & verify the current NIOSH list of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016. -

The Effects of Lidocaine and Mefenamic Acid on Post-Episiotomy
Shiraz E-Med J. 2016 March; 17(3):e36286. doi: 10.17795/semj36286. Published online 2016 March 27. Research Article The Effects of Lidocaine and Mefenamic Acid on Post-Episiotomy Pain: A Comparative Study Masoumeh Delaram,1,* Lobat Jafar Zadeh,2 and Sahand Shams3 1Faculty of Nursing and Midwifery, Shahrekord University of Medical Sciences, Shahrekord, IR Iran 2Faculty of Medicine, Shahrekord University of Medical Sciences, Shahrekord, IR Iran 3Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, IR Iran *Corresponding author: Masoumeh Delaram, Faculty of Nursing and Midwifery, Shahrekord University of Medical Sciences, Shahrekord, IR Iran. Tel: +98-3813335648, Fax: +98-3813346714, E-mail: [email protected] Received 2016 January 13; Revised 2016 February 29; Accepted 2016 March 04. Abstract Background: Most women suffer pain following an episiotomy and oral non-steroidal anti-inflammatory drugs are commonly used for pain relief. Due to the gastrointestinal side effects of oral drugs, it seems that women are more accepting of topical medications for pain relief. Objectives: Therefore, the aim of this study was to compare the effects of lidocaine and mefenamic acid on post-episiotomy pain. Patients and Methods: This clinical trial was carried out in 2011. It involved sixty women with singleton pregnancy who were given an episiotomy at 38 to 42 weeks of gestation. The participants were randomly divided into two groups. One group received 2% lido- caine cream (n = 30), while the other group received 250 mg of mefenamic acid (n = 30). The data were collected via a questionnaire and a visual analog scale. Pain intensity was compared from the first complaint by the mother and at 6, 12, and 24 hours after the delivery in both groups. -

A Proposal for the Clinical Use of Flecainide
A Proposal for the Clinical Use of Flecainide JEFFREY L. ANDERSON, MD, JAMES R. STEWART, MD, and BARRY J. CREVEY, MD Effective antiarrhythmic therapy requires a carefully sponse rate is observed in preventing induction of considered approach, including an understanding sustained ventricular tachycardia, and these pa- of the arrhythmia, the underlying cardiac disease tients should be carefully selected. Flecainide is and the drug’s pharmacokinetics. Flecainide is a promising in the treatment of supraventricular new antiarrhythmic drug that may soon be released tachycardias using atrioventricular nodal or extra- for general use. Flecainide demonstrates unsur- nodal reentrant pathways, although this use is still passed efficacy in chronic ventricular arrhythmias investigational in the United States. The drug’s use in stable patients and may become a first-choice for arrhythmias during acute myocardial infarction drug because of its ease of administration, efficacy requires further study. Flecainide possesses modest and favorable tolerance. Twice-daily dosing with negative inotropic potential. Proarrhythmic or other 100 to 200 mg usually provides effective therapy. adverse reactions have occurred primarily in set- Clinical experience suggests flecainide to be indi- tings of high drug level, poor ventricular function cated in the treatment of uniform and multiform or refractory, malignant arrhythmias, suggesting ventricular premature complexes, coupled ven- caution in these groups. tricular premature complexes, and episodes of nonsustained -

Fenestrations Control Resting-State Block of a Voltage-Gated Sodium
Fenestrations control resting-state block of a voltage- gated sodium channel Tamer M. Gamal El-Dina,1, Michael J. Lenaeusa,b,1, Ning Zhenga,c,2,3, and William A. Catteralla,2,3 aDepartment of Pharmacology, University of Washington, Seattle, WA 98195; bDivision of General Internal Medicine, Department of Medicine, University of Washington, Seattle, WA 98195; and cHoward Hughes Medical Institute, University of Washington, Seattle, WA 98195 Contributed by William A. Catterall, October 31, 2018 (sent for review September 4, 2018; reviewed by Ryan Hibbs, Michael C. Sanguinetti, and Joerg Striessnig) Potency of drug action is usually determined by binding to a specific Receptor Hypothesis posits that resting-state block is mediated receptor site on target proteins. In contrast to this conventional by drug entry from the lipid phase of the membrane into the drug paradigm, we show here that potency of local anesthetics (LAs) and receptor site in the pore, and rapid open-state block occurs as the antiarrhythmic drugs (AADs) that block sodium channels is con- drug enters the open pore from the cytoplasm (15). Both of these trolled by fenestrations that allow drug access to the receptor site forms of block are enhanced when the channel enters the inac- directly from the membrane phase. Voltage-gated sodium channels tivated state, which has high affinity for bound drug (15). It is initiate action potentials in nerve and cardiac muscle, where their well-established that LAs and AADs can reach their binding site hyperactivity causes pain and cardiac arrhythmia, respectively. LAs from the intracellular side if the activation gate is open, which and AADs selectively block sodium channels in rapidly firing nerve gives open-state block, and that inactivation increases the affinity and muscle cells to relieve these conditions.