Studies on Equine Placentitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corporate Registry Registrar's Periodical Template
Service Alberta ____________________ Corporate Registry ____________________ Registrar’s Periodical SERVICE ALBERTA Corporate Registrations, Incorporations, and Continuations (Business Corporations Act, Cemetery Companies Act, Companies Act, Cooperatives Act, Credit Union Act, Loan and Trust Corporations Act, Religious Societies’ Land Act, Rural Utilities Act, Societies Act, Partnership Act) 10024457 CANADA INC. Federal Corporation 2010182 ALBERTA LTD. Numbered Alberta Registered 2017 JAN 03 Registered Address: 10819 108 Corporation Incorporated 2017 JAN 10 Registered STREET, EDMONTON ALBERTA, T5S 2T2. No: Address: 209, 10836 - 24 STREET SE, CALGARY 2120145327. ALBERTA, T2Z 4C9. No: 2020101826. 10024503 CANADA INC. Federal Corporation 2012106 ALBERTA LTD. Numbered Alberta Registered 2017 JAN 03 Registered Address: 10819 184 Corporation Incorporated 2017 JAN 03 Registered STREET, EDMONTON ALBERTA, T5S 2T2. No: Address: 209, 10836 - 24 STREET SE, CALGARY 2120145459. ALBERTA, T2Z 4C9. No: 2020121063. 10050415 CANADA LTD. Federal Corporation 2013296 ALBERTA LTD. Numbered Alberta Registered 2017 JAN 09 Registered Address: 9831 - 107 Corporation Incorporated 2017 JAN 05 Registered STREET, WESTLOCK ALBERTA, T7P 1R9. No: Address: C/O #202, 10441 124 ST NW, EDMONTON 2120155953. ALBERTA, T5N 1R7. No: 2020132961. 101 BUFFET RESTAURANT LTD. Named Alberta 2013298 ALBERTA LTD. Numbered Alberta Corporation Incorporated 2017 JAN 13 Registered Corporation Incorporated 2017 JAN 04 Registered Address: 4909 - 50 AVENUE, BONNYVILLE Address: 2380, 10155 102 ST NW, EDMONTON ALBERTA, T9N 2H1. No: 2020168106. ALBERTA, T5J 4G8. No: 2020132987. 101287488 SASKATCHEWAN LTD. Other 2013300 ALBERTA LTD. Numbered Alberta Prov/Territory Corps Registered 2017 JAN 10 Corporation Incorporated 2017 JAN 10 Registered Registered Address: 2440 KENSINGTON ROAD NW, Address: 2070 BLACKMUD CREEK DR SW, CALGARY ALBERTA, T2N 3S1. No: 2120157843. -

Ascending Placentitis in the Mare Ascenderende Placentitis Bij De Merrie
Vlaams Diergeneeskundig Tijdschrift, 2018, 87 Review 115 Ascending placentitis in the mare Ascenderende placentitis bij de merrie J. Govaere, K. Roels, C. Ververs, M. Van de Velde, V. De Lange, I. Gerits, M. Hoogewijs, A. Van Soom Department of Reproduction, Obstetrics and Herd Health Faculty of Veterinary Medicine, Ghent University, Salisburylaan 133, B-9820 Belgium [email protected] A BSTRACT Ascending placentitis in the mare, which affects 3 to 7% of pregnancies, is a common cause of abortion, premature birth and delivery of compromised foals (Troedsson, 2003; LeBlanc, 2010). Since the infection ascends from the caudal genital tract, the first and most distinct lesions are seen near the caudal pole area of the allantochorion adjacent to the cervix. The symptoms are not always obvious or will be exhibited only at a later stage of the disease process, which renders timely adequate treatment difficult. Moreover, experimental models of placentitis in the mare are difficult to maintain and double-blind, controlled studies are scarce, making it hard to formulate clear science-based advice. In this paper, the diagnosis is discussed on the basis of the symptoms, the ultrasound examinations and the endocrinological parameters, and the therapeutic and prognostic considerations are evaluated. SAMENVATTING Een ascenderende infectie van de placenta wordt bij 3 tot 7% van de drachtige merries gezien. Het is een veel voorkomende oorzaak van abortus, premature geboorte en zwak geboren veulens (Troeds- son, 2003; LeBlanc, 2010). Gezien de infectie opklimt vanuit de caudale geslachtstractus, zijn de eerste zichtbare en meest uitgesproken letsels te vinden ter hoogte van de caudale pool van het allantochorion waar dit tegen de baarmoedermond aan ligt. -
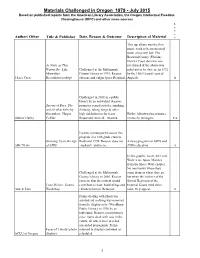
Materials Challenged in Oregon 1979
Materials Challenged in Oregon 1979 - July 2015 Based on published reports from the American Library Association, the Oregon Intellectual Freedom Clearinghouse (OIFC) and other news sources L e v e Author/ Other Title & Publisher Date, Reason & Outcome Description of Material l This rap album was the first music work to be prosecuted under obscenity law. The Broward County (Florida) District Court decision was As Nasty as They overturned & the album was Wanna Be, Luke Challenged at the Multnomah ruled not to be obscene in 1992 Skywalker County Library in 1995. Reason: by the 11th Circuit Court of 2 Live Crew Records(recording) obscene and vulgar lyrics Retained. Appeals. A Challenged in 2011 in a public library by an individual. Reason: Secrets of Boys, The promotes sexual activity, smoking, and all other titles by drinking, taking drugs & other this author; Harper high risk behavior for teens. Hailey Abbott writes romance Abbott, Hailey Collins Requested removal; retained. novels for teenagers. YA Parents challenged the use of this program in a 12th grade class in Growing Up in the Age Redmond 1994. Reason: does not A news program on AIDS and ABC News of AIDS emphasize abstinence AIDS education A In this graphic novel, the Lone Wolves are Space Marines from the Space Wolf chapter. No one knows where they Challenged at the Multnomah come from or where they go, County Library in 2005. Reason: but when the soldiers of the concern that the content would Slovak Regiment of the Lone Wolves, Games contribute to hate, bad feelings and Imperial Guard need them Abnett, Dan Workshop. -

Virgil, Aeneid 11 (Pallas & Camilla) 1–224, 498–521, 532–96, 648–89, 725–835 G
Virgil, Aeneid 11 (Pallas & Camilla) 1–224, 498–521, 532–96, 648–89, 725–835 G Latin text, study aids with vocabulary, and commentary ILDENHARD INGO GILDENHARD AND JOHN HENDERSON A dead boy (Pallas) and the death of a girl (Camilla) loom over the opening and the closing part of the eleventh book of the Aeneid. Following the savage slaughter in Aeneid 10, the AND book opens in a mournful mood as the warring parti es revisit yesterday’s killing fi elds to att end to their dead. One casualty in parti cular commands att enti on: Aeneas’ protégé H Pallas, killed and despoiled by Turnus in the previous book. His death plunges his father ENDERSON Evander and his surrogate father Aeneas into heart-rending despair – and helps set up the foundati onal act of sacrifi cial brutality that caps the poem, when Aeneas seeks to avenge Pallas by slaying Turnus in wrathful fury. Turnus’ departure from the living is prefi gured by that of his ally Camilla, a maiden schooled in the marti al arts, who sets the mold for warrior princesses such as Xena and Wonder Woman. In the fi nal third of Aeneid 11, she wreaks havoc not just on the batt lefi eld but on gender stereotypes and the conventi ons of the epic genre, before she too succumbs to a premature death. In the porti ons of the book selected for discussion here, Virgil off ers some of his most emoti ve (and disturbing) meditati ons on the tragic nature of human existence – but also knows how to lighten the mood with a bit of drag. -

Tunisian Theater at the Turn of the Century: "Hammering the Same Nail" in Jalila Baccar and Fadhel Jaïbi's Theater Rafika Zahrouni Washington University in St
Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) Spring 3-11-2014 Tunisian Theater at the Turn of the Century: "Hammering the Same Nail" in Jalila Baccar and Fadhel Jaïbi's Theater Rafika Zahrouni Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/etd Recommended Citation Zahrouni, Rafika, "Tunisian Theater at the Turn of the Century: "Hammering the Same Nail" in Jalila Baccar and Fadhel Jaïbi's Theater" (2014). All Theses and Dissertations (ETDs). 1274. https://openscholarship.wustl.edu/etd/1274 This Dissertation is brought to you for free and open access by Washington University Open Scholarship. It has been accepted for inclusion in All Theses and Dissertations (ETDs) by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Program in Comparative Literature Dissertation Examination Committee: Nancy Berg, Chair Robert Hegel Robert Henke Pascal Ifri Lynne Tatlock Tunisian Theater at the Turn of the Century: “Hammering the Same Nail” in Jalila Baccar and Fadhel Jaïbi’s Theater by Rafika Zahrouni A dissertation presented to the Graduate School of Arts and Sciences of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy May 2014 St. Louis, Missouri Copyright by Rafika Zahrouni © 2014 Table of Contents Acknowledgments ………………………………………………………………….……….. iii Introduction: From Edison Theater in St. Louis to the New Theater of Tunis ….................... 1 Chapter 1: Background of Tunisian Theater History ……………………………….…......... 17 Chapter 2: The Development of the New Theater ………………………………………….. 58 Chapter 3: From Silence to Madness, from Madness to Speech: The Psychiatric Institution as Metaphor …………………………………………………………………………………. -

Sandspur, Vol 114, No 04, October 08, 2007
University of Central Florida STARS The Rollins Sandspur Newspapers and Weeklies of Central Florida 10-8-2007 Sandspur, Vol 114, No 04, October 08, 2007 Rollins College Find similar works at: https://stars.library.ucf.edu/cfm-sandspur University of Central Florida Libraries http://library.ucf.edu This Newspaper is brought to you for free and open access by the Newspapers and Weeklies of Central Florida at STARS. It has been accepted for inclusion in The Rollins Sandspur by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rollins College, "Sandspur, Vol 114, No 04, October 08, 2007" (2007). The Rollins Sandspur. 1842. https://stars.library.ucf.edu/cfm-sandspur/1842 The andspuROLLINS COUJBGE •WINTER PARK, FLORIDr A LIFE & TIMES Read horror stories of students who were locked out throughout their college years.' PAGE 10 StSHSSHBBHHstBSKi THE STUDENT VOICE OF ROLLINS COLLEGE SINCE 1894 VOL. 114 ISSUE 04 Tvww.thesandsDur.org October 8, 2007 L] ndsay Eric Zivot played the psy ell theatre this chiatrist, Martin Dysart; he por trayed good character and stayed past \ i Tony Aware focused the whole time. Zivot got winni] its stage into character well and thafs what Howe the first tim< kept the audience entertained. the A t this horst packe<1 s ler theatre; i Alan Strang (Michael was fi rst nerformed in 1979. Tn« Nardelli) really took chances playing his part and he was openir is good as ex praised for that His character pected t of spectators reached higher limits and it did 2 in their seats come out successful. -

The Alberta Gazette, Part I, March 15, 1999
The Alberta Gazette PART 1 _______________________________________________________________________ Vol. 95 EDMONTON, MONDAY, MARCH 15, 1999 No.5 _______________________________________________________________________ PROCLAMATION [GREAT SEAL] CANADA PROVINCE OF ALBERTA H.A. “Bud” Olson, Lieutenant Governor. ELIZABETH THE SECOND, by the Grace of God, of the United Kingdom, Canada, and Her Other Realms and Territories, QUEEN, Head of the Commonwealth, Defender of the Faith P R O C L A M A T I O N To all to Whom these Presents shall come GREETING Paul Bourque, Deputy Minister of Justice and Deputy Attorney General WHEREAS section 13 of the Alberta Health Care Insurance Amendment Act, 1998 provides that that Act comes into force on Proclamation; and WHEREAS it is expedient to proclaim the Alberta Health Care Insurance Amendment Act, 1998 in force: NOW KNOW YE THAT by and with the advice and consent of Our Executive Council of Our Province of Alberta, by virtue of the provisions of the said Act hereinbefore referred to and of all other power and authority whatsoever in Us vested in that behalf, We have ordered and declared and do hereby proclaim the Alberta Health Care Insurance Amendment Act, 1998 in force on March 1, 1999. IN TESTIMONY WHEREOF We have caused these Our Letters to be made Patent and the Great Seal of Our Province of Alberta to be hereunto affixed. WITNESS: THE HONOURABLE H. A. “BUD” OLSON, Lieutenant Governor of Our Province of Alberta, in Our City of Edmonton in Our Province of Alberta, this 24 day of February in the Year of Our Lord One Thousand Nine Hundred and Ninety-nine and in the Forty-eighth Year of Our Reign. -

Preview, the Gallery Guide | June–August, 2007
CALENDAR OF OPENINGS - PG 95 GALLERY INDEX - PG 91 THE GALLERY GUIDE ALBERTA ■ BRITISH COLUMBIA ■ OREGON ■ WASHINGTON June/July/August 2007 www.preview-art.com www.vanartgallery.bc.ca 24-hour Info604662 4719 BC 750 HornbyStreet Vancouver www.vanartgallery.bc.ca America. Purchaseadvancedtimedticketsonlineat in finest collectionsof19th-and 20th-centuryEuropeanart Canadian venueontheinternational tourofonethe Art Gallery the Vancouver This summerthegreatestnamesinartwillbeat . Don’t miss the exclusive . Don’tmisstheexclusive Presenting Sponsor: Media Sponsor: Henri Fantin-Latour, Marie-Yolande de Fitz-James, 1867, (detail), oil on fabric, The Cleveland Museum of Art, Gift of Lewis C. Williams. © The Cleveland Museum of Art FORT ST. JOHN BRITISH ALBERTA COLUMBIA DAWSON CREEK PRINCE GEORGE EDMONTON QUEEN CHARLOTTE ISLANDS WEST NORTH DEEP COVE MCBRIDE VANCOUVER WELLS VANCOUVER BURNABY PORT MOODY NEW WESTMINSTER COQUITLAM VANCOUVER MISSION RICHMOND SURREY MAPLE RIDGE CHILLIWACK DELTA FORT LANGLEY ABBOTSFORD TSAWWASSEN WHITE ROCK WILLIAMS LAKE PRINCE RUPERT 100 MILE HOUSE CALGARY SALMON ARM BANFF SILVER STAR MOUNTAIN KAMLOOPS VERNON CAMPBELL RIVER WHISTLER KASLO KELOWNA COURTENAY COMOX HARRISON MEDICINE HAT UNION BAY HOT SPRINGS SUMMERLAND NELSON LETHBRIDGE SUNSHINE COAST VANCOUVER, BC PENTICTON CASTLEGAR PARKSVILLE OSOYOOS OLIVER TOFINO NANAIMO CHILLIWACK GRAND FORKS GULF ISLANDS OROVILLE DUNCAN BELLINGHAM SHAWNIGAN LAKE EASTSOUND SAANICH/SIDNEY ORCAS ISLAND TWISP LAKE COWICHAN LA CONNER SOOKE EVERETT VICTORIA FRIDAY HARBOR, SAN JUAN ISLAND PORT LANGLEY MONROE ANGELES KIRKLAND SPOKANE SEATTLE BELLEVUE TACOMA OLYMPIA WASHINGTON ASTORIA SEASIDE LONGVIEW CANNON BEACH GOLDENDALE PORTLAND MCMINVILLE SHERIDAN SALEM PACIFIC CITY OREGON EUGENE ASHLAND Serving the visual arts community since 1986 Celebrating 21 years www.preview-art.com 8 PREVIEW COVER: Anne Siems, Little Fox (2007), mixed media [Laura Russo Gallery, Portland OR, Jun 7-30] previews ALBERTA Vol. -

Clinical Chorioamnionitis at Term: New Insights Into the Etiology, Microbiology, and the Fetal, Maternal and Amniotic Cavity Inflammatory Responses
SEMMELWEIS 200 SEMMELWEIS 200 Roberto Romero1-4, Nardhy Gomez-Lopez1,5,6, Juan Pedro Kusanovic1,7, Percy Pacora1,5, Bogdan Panaitescu1,5, Offer Erez1,5, Bo H. Yoon1,8 Clinical chorioamnionitis at term: New insights into the etiology, microbiology, and the fetal, maternal and amniotic cavity inflammatory responses Roberto Romero, M.D., D.Med.Sci. 1 Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U S Department of Health and Human Services, Bethesda, Maryland, and Detroit, Michigan, USA 2 Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, Michigan, USA 3 Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, Michigan, USA 4 Center for Molecular Medicine and Genetics, Wayne State University, Detroit, Michigan, USA 5 Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA 6 Department of Immunology, Microbiology and Biochemistry, Wayne State University School of Medicine, Detroit, Michigan, USA 7 Division of Obstetrics and Gynecology, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile 8 Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Republic of Korea 2018. JÚNIUS NŐGYÓGYÁSZATI ÉS SZÜLÉSZETI TOVÁBBKÉPZŐ SZEMLE 103 SEMMELWEIS 200 Abstract Clinical chorioamnionitis is the most common infection related diagnosis made in labor and delivery units worldwide. It is traditionally believed to be due to microbial invasion of the amniotic cavity, which elicits a maternal inflammatory response characterized by maternal fever, uterine tenderness, maternal tachycardia and leukocytosis. The condition is often -as sociated with fetal tachycardia and a foul smelling amniotic fluid. -

International Viewbook
TO BE THE BEST, TRAIN WITH THE BEST! For more than 50 years, AMDA has been devoted to training the actor, singer and dancer for stage, film and television. AMDA has launched some of the most successful global careers of performers in theatre, film, television and dance. That’s because aspiring actors, singers and dancers from all over the world receive world-class training at AMDA. AMDA has two campuses—one in each of the entertainment capitals of the world: Los Angeles and New York City. AMDA College of the Performing Arts, located in the heart of Hollywood, offers Bachelor of Fine Arts (BFA) and Associate of Occupational Studies (AOS) degrees. The American Musical and Dramatic Academy, located in the prestigious Upper West Side of Manhattan, offers professional certificate programs with an option to apply to the AMDA Los Angeles campus to complete a BFA degree. 1 AMDA Facts and Figures Founded in 1964 as a musical theatre school emerging from the vibrant Manhattan arts scene in New York City Opened its Los Angeles campus located in the heart of Hollywood in 2003 A private, nonprofit institution of higher education Accredited by the National Association of Schools of Theatre (NAST) and licensed to operate by the states of New York and California Ranked #4 on Playbill Magazine’s list of colleges with the greatest number of graduates on Broadway in the 2018-19 season and #6 on the Niche.com list of Best Colleges for Performing Arts in America Four distinct BFA degree programs, three AOS degree programs, and three professional conservatory certificate programs allow students to choose their paths to success in the performing arts Partial financial support to approximately 95% of enrolled students, including international students Two Great Locations - New York City and Los Angeles are two of the most culturally and ethnically diverse cities in the United States. -

An Evaluation of Compositions for Wind Band According to Specific Criteria of Serious Artistic Merit: a Second Update
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Student Research, Creative Activity, and Performance - School of Music Music, School of 8-2011 An Evaluation of Compositions for Wind Band According to Specific Criteria of Serious Artistic Merit: A Second Update Clifford Towner University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/musicstudent Part of the Music Practice Commons, and the Other Music Commons Towner, Clifford, "An Evaluation of Compositions for Wind Band According to Specific Criteria of Serious Artistic Merit: A Second Update" (2011). Student Research, Creative Activity, and Performance - School of Music. 44. https://digitalcommons.unl.edu/musicstudent/44 This Article is brought to you for free and open access by the Music, School of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Student Research, Creative Activity, and Performance - School of Music by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. AN EVALUATION OF COMPOSITIONS FOR WIND BAND ACCORDING TO SPECIFIC CRITERIA OF SERIOUS ARTISTIC MERIT: A SECOND UPDATE by Clifford N. Towner A DOCTORAL DOCUMENT Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Doctor of Musical Arts Major: Music Under the Supervision of Professor Carolyn Barber Lincoln, Nebraska August, 2011 AN EVALUATION OF COMPOSITIONS FOR WIND BAND ACCORDING TO SPECIFIC CRITERIA OF SERIOUS ARTISTIC MERIT: A SECOND UPDATE Clifford Neil Towner, D.M.A. University of Nebraska, 201l Adviser: Carolyn Barber This study is an update to the 1978 thesis of Acton Eric Ostling, Jr. -

1 Parental-Fetal Interplay of Immune Genes Leads to Intrauterine Growth
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.26.437292; this version posted March 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Parental-fetal interplay of immune genes leads to intrauterine growth restriction Gurman Kaur1,2,16, Caroline B. M. Porter2,16, Orr Ashenberg2, Jack Lee3, Samantha J. Riesenfeld2,4, Matan Hofree2, Maria Aggelakopoulou5, Ayshwarya Subramanian2, Subita Balaram Kuttikkatte1, Kathrine E. Attfield5, Christiane A. E. Desel5,6, Jessica L. Davies5, Hayley G. Evans5, Inbal Avraham- Davidi2, Lan T. Nguyen2, Danielle A. Dionne2, Anna E. Neumann7, Lise Torp Jensen8, Thomas R. Barber1, Elizabeth Soilleux9, Mary Carrington10,11, Gil McVean12, Orit Rozenblatt-Rosen2,13, Aviv Regev2,13,14,15,*, Lars Fugger1,5,8,* 1MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Oxford, UK 2Klarman Cell Observatory, Broad Institute of MIT and Harvard, Cambridge, MA, USA 3Department of Biomedical Engineering, School of Biomedical Engineering and Imaging Sciences, King's College London, London, UK 4Pritzker School of Molecular Engineering, University of Chicago, Chicago, IL, USA 5Oxford Centre for Neuroinflammation, Nuffield Department of Clinical Neurosciences, MRC Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Oxford, UK 6Current address: University Department of Neurology, University Hospital Magdeburg,