QAPP for the Chemical Characterization of Select
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Material Safety Data Sheet
MATERIAL SAFETY DATA SHEET SRM Supplier: National Institute of Standards and Technology SRM Number: 186g Standard Reference Materials Program MSDS Number: 186g 100 Bureau Drive, Stop 2321 SRM Name: pH Standards Gaithersburg, Maryland 20899-2321 Potassium Dihydrogen Phosphate (186-I-g); Disodium Hydrogen Phosphate (186-II-g) Date of Issue: 07 November 2003 MSDS Coordinator: Carmen Davis Phone: (301) 975-6776 FAX: (301) 926-4751 ChemTrec: 1-800-424-9300 E-mail: [email protected] SECTION I. MATERIAL IDENTIFICATION Material Name: pH Standards: Potassium Dihydrogen Phosphate (186-I-g); Disodium Hydrogen Phosphate (186-II-g) Description: SRM 186g consists of two components, each prepared to ensure high purity and uniformity: KH2PO4, Potassium Dihydrogen Phosphate (186-I-g) and Na2HPO4, Disodium Hydrogen Phosphate (186-II-g). However, neither SRM component is certified for purity of substance. A unit of SRM 186g consists of 30 g of potassium dihydrogen phosphate (186-I-g) and 45 g of disodium hydrogen phosphate (186-II-g), each contained in its respective clear glass bottle. Other Designations: Potassium Dihydrogen Phosphate (potassium acid phosphate; monopotassium phosphate; potassium diphosphate; potassium biphosphate; potassium orthophosphate; potassium dihydrogen phosphate) Disodium Hydrogen Phosphate (disodium phosphate; disodium acid orthophosphate; soda phosphate; disodium phosphoric acid; disodium monohydrogen phosphate; monohydrogen disodium phosphate; DSP; sodium phosphate; sodium phosphate (Na2HPO4); hydrogen disodium phosphate; phosphoric acid, disodium salt; sodium monohydrogen phosphate; anhydrous sodium acid phosphate; disodium acid phosphate; dibasic sodium phosphate; disodium orthophosphate; disodium hydrogenorthophosphate) Name Chemical Formula CAS Registry Number Potassium Dihydrogen Phosphate KH2PO4 7778-77-0 Disodium Hydrogen Phosphate Na2HPO4 7558-79-4 DOT Classification: Potassium dihydrogen phosphate and disodium hydrogen phosphate are not regulated by DOT. -

Fatty Amines, Amidoamines, and Their Derivatives From
(19) TZZ ¥ Z_T (11) EP 2 632 890 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 69/533 (2006.01) C07C 69/593 (2006.01) 11.01.2017 Bulletin 2017/02 C11D 1/28 (2006.01) C11D 1/74 (2006.01) B01F 17/00 (2006.01) (21) Application number: 11838500.4 (86) International application number: (22) Date of filing: 25.10.2011 PCT/US2011/057602 (87) International publication number: WO 2012/061095 (10.05.2012 Gazette 2012/19) (54) FATTY AMINES, AMIDOAMINES, AND THEIR DERIVATIVES FROM NATURAL OIL METATHESIS FETTAMINE, AMIDOAMINE UND DERIVATE DAVON AUS EINER ERDÖLMETATHESE AMINES GRASSES, AMIDOAMINES GRASSES ET LEURS DÉRIVÉS PROVENANT DE LA MÉTATHÈSE D’HUILES NATURELLES (84) Designated Contracting States: • LUEBKE, Gary AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Chicago GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO IL 60660 (US) PL PT RO RS SE SI SK SM TR • LUKA, Renee Park Ridge (30) Priority: 25.10.2010 US 406570 P IL 60068 (US) 25.10.2010 US 406556 P • MALEC, Andrew, D. 25.10.2010 US 406547 P Chicago IL 60657 (US) (43) Date of publication of application: • MASTERS, Ronald, A. 04.09.2013 Bulletin 2013/36 Glenview IL 60025 (US) (73) Proprietor: Stepan Company • MUNIE, Lawrence, A. Northfield, Illinois 60093 (US) Grayslake IL 60030 (US) (72) Inventors: • MURPHY, Dennis, S. • ALLEN, Dave, R. Libertville Chicago IL 60048 (US) IL 60610 (US) • SHAPIRO, Irene •ALONSO,Marcos Buffalo Grove Chicago IL 60089 (US) IL 60660 (US) •SKELTON,Patti • BERNHARDT, Randal, J. -

Sodium Diacetate
Technical Data Sheet Ref.: 2019_12v09 Sodium Diacetate Stability Description SodiumDiacetate is stable for 3 years from Sodium Diacetate is available as a free date of production. Physical stability and flowing white crystal, containing appearance may change before the end of approximately equimolar amounts of shelf-life if not stored single-stacked in Sodium Acetate and acetic acid. closed original packaging, dry and at room temperature. PRODUCT PROPERTIES Handling Product is classified as irritant. Always Product name Sodium Diacetate check the Safety Data Sheet and label Formula CH3COONaCH3COOH before using the product. Molecular weight 142.08 g/mol CAS No. 126-96-5 Packaging EINECS No. 2048149 Sodium Diacetate crystal is packed in 900 kg big bags. HS code US 2915.29.5000 HS code EU 2915.29.00 Safety precautions Please see the Safety Data Sheet before handling the material. Product grades Sodium Diacetate has a free acetic acid content of 40% - 43%. Application Sodium Diacetate is used in haemodialysis. The product is also used in other applications where it is advantageous to use a solid source of free acetic acid, e.g. for safer handling and reduced transportation costs. Warranty. This information herein is offered as a guide and is believed to be accurate and reliable as of the date of the printing. The values given are not to be considered as a warranty and they are subject to change without prior notice. For additional information regarding our products or for information concerning current specifications, please contact our Technical Service. Niacet Corporation www.niacet.com Niacet b.v. 400, 47th Street P.O. -

Effects of Sodium Citrate Plus Sodium Diacetate and Buffered Vinegar on Escherichia Coli O157:H7 and Psychrotrophic Bacteria in Brine-Injected Beef
359 Journal of Food Protection, Vol. 74, No. 3, 2011, Pages 359–364 doi:10.4315/0362-028X.JFP-10-294 Copyright G, International Association for Food Protection Effects of Sodium Citrate plus Sodium Diacetate and Buffered Vinegar on Escherichia coli O157:H7 and Psychrotrophic Bacteria in Brine-Injected Beef AMUDHAN PONRAJAN,1,2 MARK A. HARRISON,1 JACOB R. SEGERS,2 BRADLEY K. LOWE,2 RUSSELL O. MCKEITH,2 T. DEAN PRINGLE,2 KARINA G. MARTINO,1 JAKE H. MULLIGAN,1 AND ALEXANDER M. STELZLENI2* Downloaded from http://meridian.allenpress.com/jfp/article-pdf/74/3/359/1685103/0362-028x_jfp-10-294.pdf by guest on 01 October 2021 1Department of Food Science and Technology and 2Department of Animal and Dairy Sciences, University of Georgia, Athens, Georgia 30602, USA MS 10-294: Received 15 July 2010/Accepted 4 December 2010 ABSTRACT The objective of this research was to examine the effects of sodium citrate plus sodium diacetate or buffered vinegar on Escherichia coli O157:H7 and psychrotrophic bacteria when incorporated in brine solutions for injected beef. Two experiments were conducted in which 30 top rounds and 30 top sirloins were injected (110%) to contain (i) 0.5% sodium chloride and 0.4% sodium tripolyphosphate as the control (CNT); (ii) CNT with a 1% solution of 80% sodium citrate plus 20% sodium diacetate (SCzD); or (iii) CNT with 2% buffered vinegar (VIN) in the final product. For the E. coli challenge, muscles were surface inoculated to target 6 log CFU/cm2. After injection and 10 days of storage in a vacuum package (4uC), one half of each muscle was sampled raw and the other half was cooked to an internal temperature of 60uC with a 12-min hold. -

Fatty Amines from Palm Oil and Palm Kernel Oil
SHORT COMMUNICATION JOURNALJournal of OF Oil OIL Palm PALM Research RESEARCH Vol. 23 (DECEMBER 23 December 2011) 2011 p. 1222-1226 FATTY AMINES FROM PALM OIL AND PALM KERNEL OIL WOLFGANG RUPILIUS* ABSTRACT In this article, the most important technologies to produce fatty amines from fatty acids and fatty alcohols are described. Surprisingly, none of these technologies are practised in the palm oil producing countries. While Southeast Asia became the global leader in the field of fatty acids and and fatty alcohols, the area of fatty amines has been completely ignored until now. The reasons for this abstention will be analysed, and possible concepts for a future development of this industry in the region will be evaluated. Keywords: fatty amines, palm oil, palm kernel oil, fatty alcohols. Date received: 5 May 2011; Sent for revision: 10 May 2011; Received in final form: 9 September 2011; Accepted: 15 November 2011. starting material (surfactants, biocides, flotation INTRODUCTION agents, bitumen emulsifier, corrosion inhibitor, etc.). The total global fatty amine production from Fatty amines are produced by different fatty acids and fatty alcohols is around of 800 000 t. manufacturing technologies in many countries Surprisingly none of the major palm oil and palm of the world (Table 1). There is no reliable data kernel producing countries are active in this field. concerning the global production of different The dramatic developments in fatty acid, fatty fatty amines since the amine producers are also alcohol, fatty methyl ester and glycerine production producers of derivatives using fatty amines as the in Malaysia and Indonesia have not been seen until recently in the area of fatty amines. -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -

Chemistry Module 7
CHEMISTRY MODULE 7 Organic Chemistry CHEMISTRY MODULE 7 – ORGANIC CHEMISTRY NOMENCLATURE Inquiry question: How do we systematically name organic chemical compounds? ● investigate the nomenclature of organic chemicals, up to C8, using IUPAC conventions, including simple methyl and ethyl branched chains, including: (ACSCH127) – alkanes – alkenes – alkynes – alcohols (primary, secondary and tertiary) – aldehydes and ketones – carboxylic acids – amines and amides – halogenated organic compounds ● explore and distinguish the different types of structural isomers, including saturated and unsaturated hydrocarbons, including: (ACSCH035) – chain isomers – position isomers – functional group isomers HYDROCARBONS Inquiry question: How can hydrocarbons be classified based on their structure and reactivity? ● construct models, identify the functional group, and write structural and molecular formulae for homologous series of organic chemical compounds, up to C8 (ACSCH035) : – alkanes – alkenes – alkynes ● conduct an investigation to compare the properties of organic chemical compounds within a homologous series, and explain these differences in terms of bonding (ACSCH035) ● analyse the shape of molecules formed between carbon atoms when a single, double or triple bond is formed between them ● explain the properties within and between the homologous series of alkanes with reference to the intermolecular and intramolecular bonding present ● describe the procedures required to safely handle and dispose of organic substances (ACSCH075) ● examine the environmental, -
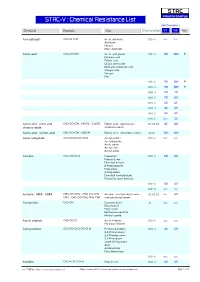
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Vaccines Chart
VACCINE CONTAINS BREAKDOWN Adenovirus human-diploid Human-diploid fibroblast cell cultures (strain WI-38) is a diploid human cell strain composed of fibroblasts derived ***Vaccine fibroblast cell from lung tissue of a 3-months gestation aborted female fetus (https://en.wikipedia.org/wiki/WI-38) indicated for cultures (strain WI- active 38), Dulbecco’s 2) Dulbecco’s Modified Eagle’s Medium is the mostly broadly suitable medium for many adherent cell phenotypes immunizatio Modified Eagle’s among defined media for cell and tissue culture. The Dulbecco’s modification is an enhanced supplementary n for the Medium, fetal formulation that boosts select amino acid and vitamin content of the original Eagle’s medium is up to fourfold. Our prevention bovine serum, selection includes a range of glucose concentrations, as well as formulations with and without L-glutamine. Products of febrile sodium bicarbonate, without the pH indicator phenol red are available for estrogen-sensitive applications, and our comprehensive offering acute monosodium includes convenient, ready-to-use liquid formats, as well as economical powdered media for easier storage and longer respiratory glutamate, sucrose, shelf life (https://www.sigmaaldrich.com/life-science/cell-culture/classical-media-salts/dmem.html) disease D-mannose, caused by Fructose, dextrose, 3) Fetal bovine serum is the liquid fraction of clotted blood from fetal calves, depleted of cells, fibrin and clotting Adenovirus human serum factors, but containing a large number of nutritional and macromolecular factors essential for cell growth. Bovine Type 4 and albumin, potassium serum albumin is the major component of FBS. Growth factors in FBS are essential for the maintenance and growth of Type 7. -

Used at Rocky Flats
. TASK 1 REPORT (Rl) IDENTIFICATION OF CHEMICALS AND RADIONUCLIDES USED AT ROCKY FLATS I PROJECT BACKGROUND ChemRisk is conducting a Rocky Flats Toxicologic Review and Dose Reconstruction study for The Colorado Department of Health. The two year study will be completed by the fall of 1992. The ChemRisk study is composed of twelve tasks that represent the first phase of an independent investigation of off-site health risks associated with the operation of the Rocky Flats nuclear weapons plant northwest of Denver. The first eight tasks address the collection of historic information on operations and releases and a detailed dose reconstruction analysis. Tasks 9 through 12 address the compilation of information and communication of the results of the study. Task 1 will involve the creation of an inventory of chemicals and radionuclides that have been present at Rocky Flats. Using this inventory, chemicals and radionuclides of concern will be selected under Task 2, based on such factors as the relative toxicity of the materials, quantities used, how the materials might have been released into the environment, and the likelihood for transport of the materials off-site. An historical activities profile of the plant will be constructed under Task 3. Tasks 4, 5, and 6 will address the identification of where in the facility activities took place, how much of the materials of concern were released to the environment, and where these materials went after the releases. Task 7 addresses historic land-use in the vicinity of the plant and the location of off-site populations potentially affected by releases from Rocky Flats. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures.