Material Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaccines Chart
VACCINE CONTAINS BREAKDOWN Adenovirus human-diploid Human-diploid fibroblast cell cultures (strain WI-38) is a diploid human cell strain composed of fibroblasts derived ***Vaccine fibroblast cell from lung tissue of a 3-months gestation aborted female fetus (https://en.wikipedia.org/wiki/WI-38) indicated for cultures (strain WI- active 38), Dulbecco’s 2) Dulbecco’s Modified Eagle’s Medium is the mostly broadly suitable medium for many adherent cell phenotypes immunizatio Modified Eagle’s among defined media for cell and tissue culture. The Dulbecco’s modification is an enhanced supplementary n for the Medium, fetal formulation that boosts select amino acid and vitamin content of the original Eagle’s medium is up to fourfold. Our prevention bovine serum, selection includes a range of glucose concentrations, as well as formulations with and without L-glutamine. Products of febrile sodium bicarbonate, without the pH indicator phenol red are available for estrogen-sensitive applications, and our comprehensive offering acute monosodium includes convenient, ready-to-use liquid formats, as well as economical powdered media for easier storage and longer respiratory glutamate, sucrose, shelf life (https://www.sigmaaldrich.com/life-science/cell-culture/classical-media-salts/dmem.html) disease D-mannose, caused by Fructose, dextrose, 3) Fetal bovine serum is the liquid fraction of clotted blood from fetal calves, depleted of cells, fibrin and clotting Adenovirus human serum factors, but containing a large number of nutritional and macromolecular factors essential for cell growth. Bovine Type 4 and albumin, potassium serum albumin is the major component of FBS. Growth factors in FBS are essential for the maintenance and growth of Type 7. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

List of Designated Additives
The Japan Food chemical Research Faundation List of Designated Additives The substances below are the designated additives appearing in Table 1, as mentioned in Article 12 of the Enforcement Regulations under the Food Sanitation Law. These additives are listed here in alphabetic order. They are 455 in total as of July 03, 2018. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese table. 16 Acesulfame Potassium(Acesulfame K) 20 Acetaldehyde 322 Acetic Acid, Glacial 23 Acetone 22 Acetophenone 17 Acetylated Distarch Adipate 19 Acetylated Distarch Phosphate 18 Acetylated Oxidized Starch 5 Adipic Acid 26 Advantame 32 DL-Alanine 190 Aliphatic Higher Alcohols 191 Aliphatic Higher Aldehydes (except those generally recognized as highly toxic) 192 Aliphatic Higher Hydrocarbons (except those generally recognized as highly toxic) 178 Allyl Cyclohexylpropionate 364 Allyl Hexanoate (Allyl Caproate) 53 Allyl Isothiocyanate (Volatile Oil of Mustard) 429 Aluminum Ammonium Sulfate (Crystal: Ammonium Alum, Desiccated: Burnt Ammonium Alum) 430 Aluminum Potassium Sulfate (Crystal: Alum or Potassium Alum, Desiccated: Burnt Alum) 29 (3-Amino-3-carboxypropyl)dimethylsulfonium chloride 43 Ammonia 35 Ammonium Alginate 240 Ammonium Bicarbonate (Ammonium Hydrogen Carbonate) 237 Ammonium Carbonate 81 Ammonium Chloride 446 Ammonium Dihydrogen Phosphate (Ammonium Phosphate, Monobasic or Monoammonium Phosphate) 44 Ammonium isovalerate 98 Ammonium Persulfate 431 Ammonium Sulfate 30 Amylalcohol -
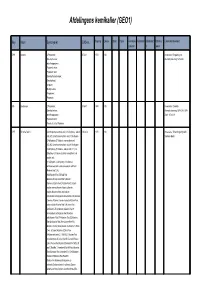
Afdelingens Kemikalier (GEO1)
Afdelingens kemikalier (GEO1) Key Navn Synonymer CAS-nr. Bygning Lokale Skab Hylde Kemikalie Købsdato Udløbsdat Batchnu Lokale kommentarer: nummer o mmer 7299 Acetone 2-Propanone; 67-64-1 1674 138 Anvendelse: Rengøring mm. Dimethyl ketone; Alternativ placering: 1672-242 beta-Ketopropane; Pyroacetic ether; Pyroacetic acid; Dimethylformaldehyde; Dimethylketal; propane; Methyl ketone; Propanone; Propanon; 535 Acetone p.a. 2-Propanone; 67-64-1 1674 138 Anvendelse: Cleanlab Dimethyl ketone; Alternativ placering: 1674-234; 1674- beta-Ketopropane; 238A; 1674-218 Pyroaceticether; Pyroacetic ether;Propanon; 1797 Alizarine Red S 2-Anthraquinonesulfonic acid, 3,4-dihydroxy-, sodium 130-22-3 1674 138 Anvendelse: Til farvning af tyndslib salt (6CI);2-Anthracenesulfonic acid, 9,10-dihydro- Stephane Bodin 3,4-dihydroxy-9,10-dioxo-, monosodium salt (8CI,9CI);2-Anthracenesulfonic acid, 9,10-dihydro- 3,4-dihydroxy-9,10-dioxo-, sodium salt (1:1);3,4- Dihydroxy-9,10-dioxo-2-anthracenesulfonic acid sodium salt; 9,10-Dihydro-3,4-dihydroxy-9,10-dioxo-2- anthracenesulfonic acid monosodium salt;Acid Mordant Red S 80; Acid Mordant Red SW;Acid Red Alizarine;Ahcoquinone Red S;Alizarin Carmine;Alizarin Red C;Alizarin Red S;Alizarin sodium monosulfonate;Alizarin sulfonate sodium;Alizarinsulfonic acid sodium salt;Alizarinsulfonsyrenatriumsalt;Alizarin S;Alizarine Carmine;Alizarine Carmine Indicator;Alizarin Red, water soluble;Alizarine Red A;Alizarine Red AS;Alizarine Red Indicator;Alizarine Red S monosodium salt;Alizarine Red S sodium salt;Alizarine Red SW;Alizarine Red SZ;Alizarine Red W;Alizarine Red WA;Alizarine Red WS; Alizarine Red for Wool;Alizarine S;Alizarine S Extra Conc. A Export;Alizarine S Extra Pure A;Alizarinsulfonate;C.I. -

Ammonium Dihydrogen Phosphate
AMMONIUM DIHYDROGEN PHOSPHATE ALPHA CHEMICALS PTY LTD Chemwatch Hazard Alert Code: 2 Chemwatch: 10050 Issue Date: 31/08/2019 Version No: 8.1.1.1 Print Date: 08/01/2020 Safety Data Sheet according to WHS and ADG requirements S.GHS.AUS.EN SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING Product Identifier Product name AMMONIUM DIHYDROGEN PHOSPHATE Chemical Name ammonium phosphate, monobasic H6-N-O4-P; (NH4)H2PO4; ammonium dihydrogen orthophosphate; ammonium acid phosphate; ammonium biphosphate; ammonium dihydrogen phosphate; ammonium phosphate; ammonium phosphate, primary; monoammonium dihydrogen phosphate; monoammonium phosphate; Synonyms phosphoric acid, monoammonium salt; primary ammonium phosphate; Redox MOAMM030; M.A.P.; mono ammonium phosphate, monobasic; MAP Chemical formula H6NO4P Other means of identification Not Available CAS number 7722-76-1 Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses Used as a flameproofing agent, a fertilizer, food additive and in fermentations (yeast preparations). Prevents afterglow in matches. Details of the supplier of the safety data sheet Registered company name ALPHA CHEMICALS PTY LTD Address 4 ALLEN PLACE WETHERILL PARK NSW 2099 Australia Telephone 61 (0)2 9982 4622 Fax Not Available Website ~ Email [email protected] Emergency telephone number Association / Organisation ALPHA CHEMICALS PTY LTD Emergency telephone 61 (0)418 237 771 numbers Other emergency telephone Not Available numbers SECTION 2 HAZARDS -

QAPP for the Chemical Characterization of Select
HF Project #10 Revision No. 1 October 10, 2012 Page i of 59 QUALITY ASSURANCE PROJECT PLAN FOR THE CHEMICAL CHARACTERIZATION OF SELECT CONSTITUENTS RELEVANT TO HYDRAULIC FRACTURING U. S. ENVIRONMENTAL PROTECTION AGENCY OFFICE OF RESEARCH AND DEVELOPMENT NATIONAL EXPOSURE RESEARCH LABORATORY ENVIRONMENTAL SCIENCES DIVISION October 18, 2012 APPROVED BY: ____________________/s/____________________________ __10/18/2012________ Brian Schumacher, Branch Chief, Technical Research Lead Date ___________________/s/________________________________ __10/18/2012________ Patrick DeArmond, Principal Investigator Date ___________________/s/________________________________ __10/18/2012________ Charlita Rosal, Principal Investigator Date ___________________/s/________________________________ __10/18/2012________ Georges-Marie Momplaisir, Principal Investigator Date ___________________/s/________________________________ __10/18/2012________ Ed Heithmar, Branch Quality Assurance Representative Date ___________________/s/________________________________ __10/18/2012________ George Brilis, ESD Quality Assurance Manager Date Chemical Characterization Revision No. 1 October 18, 2012 Page ii of 57 This page intentionally left blank ii Chemical Characterization Revision No. 1 October 18, 2012 Page iii of 57 TABLE OF CONTENTS SECTION A. PROJECT MANAGEMENT ............................................................................................................. 1 A3 Distribution List ............................................................................................................................................. -

Safety Assessment of Phosphoric Acid and Simple Salts As Used in Cosmetics
Safety Assessment of Phosphoric Acid and Simple Salts as Used in Cosmetics Status: Tentative Report for Public Comment Release Date: April 12, 2016 Panel Date: June 6-7, 2016 All interested persons are provided 60 days from the above date to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Director, Dr. Lillian J. Gill. The 2016 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, D.P.A. This report was prepared by Wilbur Johnson, Jr., M.S., Senior Scientific Analyst, Ivan Boyer, Ph.D., Toxicologist, and Bart Heldreth, Ph.D., Chemist. © Cosmetic Ingredient Review 1620 L STREET, NW, SUITE 1200 ◊ WASHINGTON, DC 20036-4702 ◊ PH 202.331.0651 ◊ FAX 202.331.0088 ◊ [email protected] INTRODUCTION The safety of the following 31 ingredients and their salts as used -

OPPT) US Environmental Protection Agency (7401M) 1200 Pennsylvania Avenue, N.W
January 29, 2019 Document Control Officer (DCO) Office of Pollution Prevention and Toxics (OPPT) US Environmental Protection Agency (7401M) 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 SUBJECT: Additional Information for P-18-0257 Everris NA Inc. wishes to submit to EPA responses to the agency’s review of the PMN substance under P- 18-0257. We respectfully request the agency to further consider these responses which are provided with additional information to support P-18-0257. Hi-Peak is a double salt crystal with the empirical formula K3H3(PO4)2, composed of monopotassium phosphate (MKP - KH2PO4) and dipotassium phosphate (DKP - K2HPO4) salts. During its intended use, once dissolved in water, Hi-Peak dissociates behaving as a mixture of two known, TSCA listed compounds: monopotassium phosphate (MKP - KH2PO4) (CAS No. 7778-77-0) and dipotassium phosphate (DKP - K2HPO4) (CAS No. 7758-11-4). These two compounds, Monopotassium Phosphate (MKP) and Dipotassium Phosphate (DKP), are known as common fertilizers that are highly soluble in water and are applied to plants through watering after dissolving in water (terms P-25 and P-26 of AAPFCO, official edition, 2002). The intended use of the PMN substance as an element in Hi-Peak will be applied as an inorganic fertilizer through watering as well. In addition to its use as a fertilizer, monopotassium phosphate (MKP - KH2PO4) and dipotassium phosphate (DKP - K2HPO4) salts are used as food additives. We refer to listings found in the FCC (Food Chemicals Codex and (EU) No 231/2012: E 340 (i) monopotassium phosphate (MKP); E 340 (ii) dipotassiumphosphate (DKP). -
Nomenclature of Phosphorus-Containing Compounds of Biochemical Importance (Recommendations 1976)*
Proc. Nati. Acad. Sci. USA Vol. 74, No. 6, pp. 2222-2230, June 1977 Biochemistry Nomenclature of phosphorus-containing compounds of biochemical importance (Recommendations 1976)* IUPAC-IUB COMMISSION ON BIOCHEMICAL NOMENCLATUREt The IUPAC Commissions on the Nomenclature of Inorganic 2. Phosphate may be used for "(dihydrogen phosphate)," and of Organic Chemistry (CNIC and CNOC) have recently "(disodium phosphate)," etc., (a) if the nature of the counter- provided, in the "D-Rules" (1), recommendations for naming ions is not known or is of no importance in the context, or (b) a large number of organic compounds containing phosphorus. if a mixture of ionic forms (free acid and/or monoanion and/or Many such compounds are extremely important in biochem- dianion) is in question. Thus, in most biochemical or biological istry and hence in nearly all branches of biology and medicine. systems, where the pH is around 7, "glucose phosphate" may Most of the biochemically important ones are esters and/or be used in place of "glucose dihydrogen phosphate," the proper anhydrides of various phosphorus-containing acids with com- names for the protonated form. plex organic alcohols and organic acids. Strict application of the Comments. (i) Although glucose phosphate is an ester, the "D Rules" (1) to such compounds would result, in many cases, term "phosphate ester" should not be used: "phosphoric ester" in rather complicated names, and these would be inconvenient is the appropriate generic term. (ii) When "phosphoric" is for most biochemists and biologists to use. followed by a generic term (e.g., ester, amide, group), the word However, other systems of nomenclature, in use in the bio- "acid" need not intervene. -

Calcium Chloride in the "Calcium Chloride Process" (21 CFR 184.1191)
Phosphates Handling/Processing 1 2 Identification of Petitioned Substances 3 This report addresses the following phosphate salts allowed under the National Organic Program (NOP) 4 regulations at 7 CFR 205.605(b): calcium phosphates (monobasic, dibasic and tribasic), potassium 5 phosphate, sodium acid pyrophosphate, and sodium phosphates. Chemical identifications of these 6 phosphates are included in Table 1. 7 8 Table 1: Chemical Identification of the Phosphates Listed at 7 CFR 205.605(b). Chemical Names Chemical Formula CAS Nos. E/INS No. Calcium phosphate, monobasic Calcium dihydrogen phosphate Ca(H2PO4)2 (anhydrous) 7758-23-8 Calcium biphosphate Calcium bis(dihydrogen phosphate) E 341(i) Monocalcium phosphate Primary calcium phosphate Ca(H2PO4)2 · 1 H2O 10031-30-8 Acid calcium phosphate Calcium diorthophosphate Calcium phosphate, dibasic CaHPO4 (anhydrous) 7757-93-9 Calcium hydrogen phosphate E 341(ii) Monocalcium acid phosphate CaHPO4· 2 H2O 7789-77-7 Dicalcium orthophosphate Calcium phosphate, tribasic Tricalcium diphosphate Ca3(PO4)2 (anhydrous) 7758-87-4 E 341(iii) Tricalcium phosphate Tricalcium orthophosphate Dipotassium phosphate (anhydrous) Dipotassium hydrogen phosphate K2HPO4 (anhydrous) 7758-11-4 Potassium hydrogen phosphate E 340(ii) Potassium dibasic phosphate K2HPO4· 3 H2O 16788-57-1 Potassium phosphate dibasic Sodium acid pyrophosphate (SAPP) Disodium diphosphate Na2H2P2O7 (anhydrous) 7758-16-9 E 450(vi) Disodium dihydrogen pyrophosphate; Diphosphoric acid, disodium salt Monosodium phosphate 7558-80-7 NaH2PO4 (anhydrous) -

Material Safety Data Sheet
Date of Issue: 14 February 2014 SAFETY DATA SHEET 1. SUBSTANCE AND SOURCE IDENTIFICATION Product Identifier SRM Number: 194a SRM Name: Ammonium Dihydrogen Phosphate Other Means of Identification: Not applicable. Recommended Use of This Material and Restrictions of Use This Standard Reference Material (SRM) is intended primarily for use in the fertilizer industry as a working standard in the determination of ammoniacal nitrogen and phosphorus. A unit of SRM 194a consists of 90 g of crystalline ammonium dihydrogen phosphate in a single glass bottle. Company Information National Institute of Standards and Technology Standard Reference Materials Program 100 Bureau Drive, Stop 2300 Gaithersburg, Maryland 20899-2300 Telephone: 301-975-2200 Emergency Telephone ChemTrec: FAX: 301-948-3730 1-800-424-9300 (North America) E-mail: [email protected] +1-703-527-3887 (International) Website: http://www.nist.gov/srm 2. HAZARDS IDENTIFICATION Classification Physical Hazard: Not classified. Health Hazard: Serious eye damage/Eye irritation Category 2B Label Elements Symbol No symbol Signal Word Warning Hazard Statement(s): H320 Causes eye irritation. Precautionary Statement(s): P264 Wash hands thoroughly after handling. 280 Wear protective gloves, protective clothing, eye protection. P305+P351+P338 If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P337+P313 If eye irritation persists: Get medical advice/attention. Hazards Not Otherwise Classified: Not applicable. Ingredients(s) with Unknown Acute Toxicity: Not applicable. 3. COMPOSITION AND INFORMATION ON HAZARDOUS INGREDIENTS Substance: Ammonium dihydrogen phosphate Other Designations: Ammonium biphosphate; ammonium dihydrogen orthophosphate; ammonium phosphate, monobasic; phosphoric acid; monoammonium salt. -

Physical Properties of Chemicals in PAC Revision 27 Listing
LLNL-TR-625492 Physical Properties of Chemicals in PAC Revision 27 Listing M. A. Johnson March 8, 2013 Disclaimer This document was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor Lawrence Livermore National Security, LLC, nor any of their employees makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or Lawrence Livermore National Security, LLC. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or Lawrence Livermore National Security, LLC, and shall not be used for advertising or product endorsement purposes. This work performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. Physical Properties of Chemicals in PAC Revision 27 Listing 1 Purpose The purpose of this chemical physical property listing is to provide data required to apply the DOE SCAPA Protective Action Criteria (PAC) values to calculation of the LLNL Quantity (Q) Value thresholds for facility chemical hazard classification. This chemical physical property listing based on the DOE SCAPA Protective Action Criteria (PAC) Revision 27 listing Identifies: 1. Physical state at 25°C (i.e.