Safety Assessment of Phosphoric Acid and Simple Salts As Used in Cosmetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potassium Cyanide Broth Base W/O KCN M936
Potassium Cyanide Broth Base w/o KCN M936 Potassium Cyanide Broth Base with KCN supplementation is used for the differentiation of the members of Enterobacteriaceae on the basis of potassium cyanide tolerance. Composition** Ingredients Gms / Litre Proteose peptone 3.000 Disodium phosphate 5.640 Monopotassium phosphate 0.225 Sodium chloride 5.000 Final pH ( at 25°C) 7.6±0.2 **Formula adjusted, standardized to suit performance parameters Directions Suspend 13.86 grams in 1000 ml distilled water. Heat if necessary to dissolve the medium completely. Dispense in 100 ml amounts and sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to room temperature and aseptically add sterile 1.5 ml of 0.5% potassium cyanide solution to each 100 ml of basal medium. Mix thoroughly and dispense in 1 ml amounts.Caution : Being fatally toxic extreme care should be taken while handling potassium cyanide solution. Never mouth- pipette potassium cyanide solution. Principle And Interpretation One of the many tests employed for the identification of bacteria includes the ability of an organism to grow in the presence of cyanide (1). Potassium Cyanide Broth Base is used for the differentiation of members of Enterobacteriaceae on the basis of Potassium Cyanide tolerance. Potassium Cyanide Broth Base was originally formulated by Moeller (2) and Kauffman and Moeller (3). This medium was later modified by Edwards and Ewing (4) and Edwards and Fife (5). Proteose peptone provides nitrogenous compounds, sulphur, trace elements essential for growth. Phosphates buffer the medium. Sodium chloride maintains osmotic equilibrium. Potassium cyanide inhibits many bacteria including Salmonella , Shigella and Escherichia , while members of the Klebsiella , Citrobacter , and Proteus groups grow well. -

Material Safety Data Sheet
MATERIAL SAFETY DATA SHEET SRM Supplier: National Institute of Standards and Technology SRM Number: 186g Standard Reference Materials Program MSDS Number: 186g 100 Bureau Drive, Stop 2321 SRM Name: pH Standards Gaithersburg, Maryland 20899-2321 Potassium Dihydrogen Phosphate (186-I-g); Disodium Hydrogen Phosphate (186-II-g) Date of Issue: 07 November 2003 MSDS Coordinator: Carmen Davis Phone: (301) 975-6776 FAX: (301) 926-4751 ChemTrec: 1-800-424-9300 E-mail: [email protected] SECTION I. MATERIAL IDENTIFICATION Material Name: pH Standards: Potassium Dihydrogen Phosphate (186-I-g); Disodium Hydrogen Phosphate (186-II-g) Description: SRM 186g consists of two components, each prepared to ensure high purity and uniformity: KH2PO4, Potassium Dihydrogen Phosphate (186-I-g) and Na2HPO4, Disodium Hydrogen Phosphate (186-II-g). However, neither SRM component is certified for purity of substance. A unit of SRM 186g consists of 30 g of potassium dihydrogen phosphate (186-I-g) and 45 g of disodium hydrogen phosphate (186-II-g), each contained in its respective clear glass bottle. Other Designations: Potassium Dihydrogen Phosphate (potassium acid phosphate; monopotassium phosphate; potassium diphosphate; potassium biphosphate; potassium orthophosphate; potassium dihydrogen phosphate) Disodium Hydrogen Phosphate (disodium phosphate; disodium acid orthophosphate; soda phosphate; disodium phosphoric acid; disodium monohydrogen phosphate; monohydrogen disodium phosphate; DSP; sodium phosphate; sodium phosphate (Na2HPO4); hydrogen disodium phosphate; phosphoric acid, disodium salt; sodium monohydrogen phosphate; anhydrous sodium acid phosphate; disodium acid phosphate; dibasic sodium phosphate; disodium orthophosphate; disodium hydrogenorthophosphate) Name Chemical Formula CAS Registry Number Potassium Dihydrogen Phosphate KH2PO4 7778-77-0 Disodium Hydrogen Phosphate Na2HPO4 7558-79-4 DOT Classification: Potassium dihydrogen phosphate and disodium hydrogen phosphate are not regulated by DOT. -

Safety Data Sheet TSP - TRISODIUM PHOSPHATE
Safety Data Sheet TSP - TRISODIUM PHOSPHATE Date of Revision: 2/11/2015 Section 1 – Chemical Product and Company Identification Product/Chemical Name: Trisodium Phosphate dodecahydrate Chemical Formula: Na3PO4*12H2O CAS Number: 10101-89-0 Other Designations: TSP; trisodium orthophosphate; tribasic; tertiary sodium phosphate; trisodium phosphate Derivation: Prepared by combining proper proportions of phosphoric acid and soda to form disodium phosphate, then adding a caustic soda Supplied by: PRO Chemical & Dye 126 Shove Street Fall River, MA 02724 Emergency Telephone Numbers: 800-255-3924 ChemTel. (United States) + 1 01 813-248-0585 (Outside the United States) 1. Section 2 - Hazards Identification HMIS ***** Emergency Overview ***** H 3 MAY CAUSE EYE INJURY. CAUSES SKIN IRRITATlON. MAYBE HARMFUL IF SWALLOWED. F 0 Potential Health Effects R 0 PPE Primary Entry Routes: Inhalation, ingestion or skin contact. Sec. 8 Target Organs: Skin, digestive tract. HAZARDS IDENTIFICATION Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA DCS) Skin corrosion (Category I B). H314 Serious eye damage (Category I). H318 GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe dust or mist. P264 Wash skin thoroughly after handling. P280 Wear protective gloves protective clothing/ eye protection/ face protection. P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting. P303 + P36l + P353 IF ON SKIN (or hair): Remove. Take off immediately all contaminated clothing. Rinse skin with water / shower. P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

Brochure-Product-Range.Pdf
PRODUCT RANGE 2015 edition ANSI Standard 60 NSF® CERTIFIED HALAL M ISLAMIC FOOD AND NUTRITION ® COUNCIL OF AMERICA Rue Joseph Wauters, 144 ISO 9001:2008 (Quality) / OHSAS 18001:2007 (Health/ B-4480 Engis Safety) / ISO 14001:2004 (Environment) / ISO 22000:2005 www.globulebleu.com (Food Safety) / FSSC 22000:2013 (Food Safety). Tel. +32 (0) 4 273 93 58 Our food grade phosphates are allergen free, GMO free, Fax. +32 (0) 4 275 68 36 BSE/TSE free. www.prayon.com mail. [email protected] Design by www.prayon.com PRODUCT RANGE | 11 TABLE OF CONTENTS HORTICULTURE APPLICATIONS HORTIPRAY® RANGE FOR HORTICULTURE* FOOD AND INDUSTRIAL APPLICATIONS PRODUCT NAME Bulk density P O pH N-NH Made 2 5 4 MONOAMMONIUM PHOSPHATE - NH4H2PO4 in 3 3 % 1% % Sodium orthophosphates ................................................................................... 03 g/cm lbs/ft indicative indicative indicative Water-soluble fertilisers. Sodium pyrophosphates .................................................................................... 04 HORTIPRAY® MAP Horticultural Grade 0.9 56 61 4.5 12 Sodium tripolyphosphates ................................................................................. 05 HORTIPRAY® MAP 12.60 Horticultural Grade 0.9 56 60 5 12.1 Water-soluble fertilisers; Sodium polyphosphates ..................................................................................... 06 HORTIPRAY® MAP anticalc Horticultural Grade 0.9 56 61 4.5 12 preventive action against clogging. Potassium orthophosphates ............................................................................. -

Ionic Liquid + Biomolecule
Sónia Isabel Pereira Branco Licenciatura em Ciências da Engenharia Química e Bioquímica Aqueous Biphasic System based on Cholinium Ionic Liquids: Extraction of Biologically Active Phenolic Acids Dissertação para obtenção do Grau de Mestre em Engenharia Química e Bioquímica Orientador: Doutora Isabel Maria Delgado Jana Marrucho Ferreira, Investigadora Coordenadora, Laboratório de Termodinâmica Molecular, ITQB-UNL Presidente: Doutora Susana Filipe Barreiros Arguente: Doutor Alexandre Babo de Almeida Paiva Vogal: Doutora Isabel Maria Delgado Jana Marrucho Ferreira Setembro 2014 II UNIVERSIDADE NOVA DE LISBOA Faculdade de Ciências e Tecnologia Departamento de Química Aqueous Biphasic System based on Cholinium Ionic Liquids: Extraction of Biologically Active Phenolic Acids Sónia Isabel Pereira Branco Dissertação apresentada na Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa para obtenção do grau Mestre em Engenharia Química e Bioquímica Orientadores: Doutora Isabel Maria Delgado Jana Marrucho Ferreira 2014 III IV Aqueous Biphasic Systems based on Cholinium Ionic Liquids: Extraction of Biologically Active Phenolic Acids COPYRIGHT Sónia Isabel Pereira Branco Faculdade de Ciências e Tecnologia Universidade Nova de Lisboa A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. V VI Agradecimentos Durante a realização desta tese, contei com o apoio de várias pessoas sem as quais não teria concluído esta etapa. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

Potassium Phosphate (Dibasic)
G-Biosciences, St Louis, MO, USA | 1-800-628-7730 | 1-314-991-6034 | [email protected] A Geno Technology, Inc. (USA) brand name Safety Data Sheet Cat. # RC-082 Potassium Phosphate (Dibasic) Size: 1kg think proteins! think G-Biosciences! www.GBiosciences.com Potassium Phosphate (Dibasic) Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 Revision date: 5/11/2017 Version: 1.1 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name : Potassium Phosphate (Dibasic) Chemical name : Potassium Phosphate (Dibasic) EC-No. : 231-834-5 CAS-No. : 7758-11-4 Product code : 316P Type of product : Pure substance,Hygroscopic substance. Preventive measures apply to the substance in dry state only Formula : K2HPO4 Synonyms : dibasic potassium phosphate, anhydrous / dikalium phosphate, anhydrous / dipotassium hydrogen phosphate, anhydrous / dipotassium hydrogenorthophosphate, anhydrous / dipotassium monohydrogen phosphate, anhydrous / dipotassium monophosphate, anhydrous / dipotassium orthophosphate, anhydrous / dipotassium phosphate, anhydrous / dipotassium-o-phosphate, anhydrous / DKP, anhydrous / hydrogen dipotassium phosphate, anhydrous / orthophosphate dipotassium, anhydrous / phosphoric acid, dipotassium salt / phosphoric acid, dipotassium salt, anhydrous / potassium biphosphate, anhydrous / potassium dibasic phosphate, anhydrous / potassium hydrogen phosphate(=di potassium hydrogen phosphate) -

General Properties of the Alkaline Phosphates: - Major Food and Technical Applications
Phosphorus Research Bulletin Vol. 15 (2004) p. 85-94 General Properties of the Alkaline Phosphates: - Major Food and Technical Applications P.HOURANT Deputy Business Line Manager, Prayon S.A., Business Unit Phosphates, Rue Joseph Wauters, 144 4480 Engis, Belgium; E-mail: [email protected] INTRODUCTION The alkaline phosphates are used for many food and technical applications. Phosphates have two characteristics that explain their four main properties: buffer agent, sequestering power, dispersing power and water holding capability. Those properties allow phosphates to be used in many food and technical applications. The main food applications are meat and seafood processing, baking and processed cheese, but others such as cereals, French fries, fruits and vegetables, beverages, noodles and so on also may need the use of phosphates. On the technical side, the main applications are the detergent products, the water treatment and the metal treatment. As for the food, many other applications require phosphates such as ceramics, bone china, paper and paints,... In meat products, phosphates salts interact in a unique way to bind water with proteins and improve the tenderness in meats. Treated products will maintain their juicy appearance as well as their natural nutritional properties texture and colour. In fish and seafood products, phosphates salts allow the retention of the natural juices of frozen fish fillets, prawns, shrimps, scallops and other seafood. Phosphates also help prevent the build-up of struvite crystals in tinned tuna and crabmeat. In processed cheese, phosphates are crucially important in the production of processed cheese. These products ensure a homogeneous and uniform melt of raw cheese and product stability. -

IFAC Summary of Phosphate Citations the International Food Additives
IFAC Summary of Phosphate Citations The International Food Additives Council (IFAC) is a global association representing manufacturers of food ingredients, including phosphates used as food additives. IFAC strives for the harmonization of food additive standards and specifications worldwide, and supports regulatory processes to identify, categorize and document the safety of food additives. Phosphorus is an essential element critical for several key biochemical processes in the body, including development of cell membranes, growth of bones and teeth, maintenance of acid-base balance, and cellular energetics. Phosphorus is naturally occurring in various types of foods, including meat, grains, and dairy. Additionally, inorganic phosphates can be added to foods to improve texture, flavor, shelf life, and other technological functions. Inorganic phosphates are salts or esters of phosphoric acid. Phosphoric acid is produced starting with naturally-occurring phosphate ore mined around the world. As phosphoric acid, it can be combined with other elements such as calcium, potassium, and sodium into "salts." Phosphate additives are contained in a large number of processed foods and beverages and help contribute to the vast food supply while also minimizing food waste. Following is a comprehensive list of phosphates that are approved for use in food. All of these phosphates have either been approved by the US Food and Drug Administration (FDA) as a direct food additive or reviewed by FDA and determined to be generally recognized as safe (GRAS). Also included are the CAS numbers, International Numbering System (INS) numbers, Food Chemicals Codex (FCC) references and Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluations, as available. -

Phase-Matched Metamaterials for Second-Harmonic Generation
Anna Vesala PHASE-MATCHED METAMATERIALS FOR SECOND-HARMONIC GENERATION Faculty of Engineering and Natural Sciences (ENS) Bachelor of Science Thesis April 2020 i ABSTRACT Anna Vesala: Phase-matched Metamaterials for Second-harmonic Generation Bachelor of Science Thesis Tampere University Bachelors Degree Programme in Science and Engineering Major: Physics Examiners: Dr. Mikko Huttunen and M.Sc. Timo Stolt April 2020 Metamaterials exhibit unconventional electromagnetic properties that cannot be found in nature, such as negative index of refraction or strong optical activity. Moreover, they show promise for enabling nanoscale nonlinear optics. Current nonlinear optical interactions of practical use rely on phase matching combined with long propagation lengths, which are not compatible with the size requirements of miniaturized systems. In order to be able to improve the realizable conversion efficiencies of nonlinear processes and discover novel functionalities at the nanoscale, new kinds of nonlinear metamaterials need to be investigated. By utilizing local-field enhancements and the phase engineering of localized surface plas- mon resonances, it is possible to construct metamaterials which generate nonlinear frequencies into the direction where the fundamental light came from. In this Thesis, we demonstrate how phase matching is achieved in nanoscale nonlinear materials. Especially, we fabricate three- dimensional plasmonic metamaterial devices that were phase matched for back-propagating sec- ond harmonic-generation. Our samples consist of one to five metasurfaces stacked on top of each other and the aim was to observe how the intensity of the second-harmonic field varies with the number of metasurfaces stacked in a backward phase-matched metamaterial. The results show that the second harmonic signal depends quadratically on the number of metasurfaces, which confirms that the sample was successfully phase-matched by controlling the dimensions of the nanoparticles and the separation between the metasurfaces. -
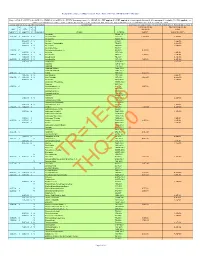
Composite Worker Air RSL May 2021 HQ10
Regional Screening Level (RSL) Composite Worker Ambient Air Table (TR=1E-06, HQ=1) May 2021 Key: I = IRIS; P = PPRTV; O = OPP; A = ATSDR; C = Cal EPA; X = PPRTV Screening Level; H = HEAST; W = TEF applied; E = RPF applied; G = user's guide Section 5; M = mutagen; V = volatile; R = RBA applied ; c = cancer; n = noncancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; SSL values are based on DAF=1; m = ceiling limit exceeded; s = Csat exceeded. Toxicity and Chemical-specific Information Contaminant Carcinogenic Target Risk (TR) = 1E-06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1E-06 THI=1 (ug/m3)-1 y (mg/m3) y l mutagen Analyte CAS No. (ug/m3) (ug or fibers/m3) Acephate 30560-19-1 2.2E-06 I 9.0E-03 I V Acetaldehyde 75-07-0 5.6E+00 3.9E+01 Acetochlor 34256-82-1 3.1E+01 A V Acetone 67-64-1 1.4E+05 2.0E-03 X Acetone Cyanohydrin 75-86-5 8.8E+00 6.0E-02 I V Acetonitrile 75-05-8 2.6E+02 V Acetophenone 98-86-2 1.3E-03 C Acetylaminofluorene, 2- 53-96-3 9.4E-03 2.0E-05 I V Acrolein 107-02-8 8.8E-02 1.0E-04 I 6.0E-03 I M Acrylamide 79-06-1 1.2E-01 2.6E+01 1.0E-03 I V Acrylic Acid 79-10-7 4.4E+00 6.8E-05 I 2.0E-03 I V Acrylonitrile 107-13-1 1.8E-01 8.8E+00 6.0E-03 P Adiponitrile 111-69-3 2.6E+01 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb Sulfone 1646-88-4 Aldicarb sulfoxide 1646-87-3 4.9E-03 I V Aldrin 309-00-2 2.5E-03 1.0E-04 X V Allyl Alcohol 107-18-6 4.4E-01 6.0E-06 C 1.0E-03 I V Allyl Chloride 107-05-1 2.0E+00 4.4E+00 5.0E-03 P Aluminum 7429-90-5 2.2E+01 Aluminum Phosphide 20859-73-8 -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5