General Properties of the Alkaline Phosphates: - Major Food and Technical Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet TSP - TRISODIUM PHOSPHATE
Safety Data Sheet TSP - TRISODIUM PHOSPHATE Date of Revision: 2/11/2015 Section 1 – Chemical Product and Company Identification Product/Chemical Name: Trisodium Phosphate dodecahydrate Chemical Formula: Na3PO4*12H2O CAS Number: 10101-89-0 Other Designations: TSP; trisodium orthophosphate; tribasic; tertiary sodium phosphate; trisodium phosphate Derivation: Prepared by combining proper proportions of phosphoric acid and soda to form disodium phosphate, then adding a caustic soda Supplied by: PRO Chemical & Dye 126 Shove Street Fall River, MA 02724 Emergency Telephone Numbers: 800-255-3924 ChemTel. (United States) + 1 01 813-248-0585 (Outside the United States) 1. Section 2 - Hazards Identification HMIS ***** Emergency Overview ***** H 3 MAY CAUSE EYE INJURY. CAUSES SKIN IRRITATlON. MAYBE HARMFUL IF SWALLOWED. F 0 Potential Health Effects R 0 PPE Primary Entry Routes: Inhalation, ingestion or skin contact. Sec. 8 Target Organs: Skin, digestive tract. HAZARDS IDENTIFICATION Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA DCS) Skin corrosion (Category I B). H314 Serious eye damage (Category I). H318 GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe dust or mist. P264 Wash skin thoroughly after handling. P280 Wear protective gloves protective clothing/ eye protection/ face protection. P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting. P303 + P36l + P353 IF ON SKIN (or hair): Remove. Take off immediately all contaminated clothing. Rinse skin with water / shower. P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

FLEET® Enemas Are Designed for Quick, Convenient Administration by Nurse, Patient Or Caregiver According to Instructions
Reference Safety Information – Fleet Enema FLEET® ENEMA, A SALINE LAXATIVE FLEET® ENEMA EXTRA®, A SALINE LAXATIVE FLEET® PEDIA-LAX® ENEMA, A SALINE LAXATIVE, FLEET® ENEMA FOR CHILDREN, A SALINE LAXATIVE FLEET® enemas are designed for quick, convenient administration by nurse, patient or caregiver according to instructions. Each is disposable after a single use. COMPOSITION: FLEET® ENEMA: Each FLEET® Enema unit, with a 2-inch, pre-lubricated Comfortip®, contains 4.5 fl. oz. (133 mL) of enema solution in a ready-to-use squeeze bottle which is not made with natural rubber latex. Each enema unit delivers a dose of 118 mL, which contains 19 g monobasic sodium phosphate monohydrate and 7 g dibasic sodium phosphate heptahydrate. Each Fleet® Enema 118 mL delivered dose contains 4.4 grams sodium. FLEET® ENEMA EXTRA®: Each FLEET® Enema EXTRA® unit, with a 2-inch, pre-lubricated Comfortip®, contains 7.8 fl. oz. (230 mL) of enema solution in a ready-to-use squeeze bottle which is not made with natural rubber latex. Each enema unit delivers a dose of 197 mL, which contains 19 g monobasic sodium phosphate monohydrate and 7 g dibasic sodium phosphate heptahydrate. Each Fleet® Enema EXTRA® 197 mL delivered dose contains 4.4 grams sodium. FLEET® PEDIA-LAX® ENEMA and FLEET® ENEMA FOR CHILDREN: Each Fleet® Pedia- Lax® Enema and FLEET® Enema for Children unit, with a 2-inch, pre-lubricated Comfortip®, contains 2.25 fl. oz. (66 mL) of enema solution in a ready-to use squeeze bottle which is not made with natural rubber latex. Each enema unit delivers a dose of 59 mL, which contains 9.5 g monobasic sodium phosphate monohydrate and 3.5 g dibasic sodium phosphate heptahydrate. -

Planning for Seafood Freezing
TTTTTTTTTTT Planning for Seafood Freezing Edward KOLBE Donald KRAMER MAB-60 2007 Alaska Sea Grant College Program University of Alaska Fairbanks Fairbanks, Alaska 99775-5040 (888) 789-0090 Fax (907) 474-6285 www.alaskaseagrant.org TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT Elmer E. Rasmuson Library Cataloging-in-Publication Data: Kolbe, Edward. Planning for seafood freezing ⁄ Edward Kolbe and Donald Kramer. – Fairbanks, Alaska : Alaska Sea Grant College Program, University of Alaska Fairbanks, 2007 126 p. : 51 ill. ; cm. (Alaska Sea Grant College Program, University of Alaska Fairbanks ; MAB-60) Includes bibliographical references and index. 1. Frozen seafood—Preservation—Handbooks, manuals, etc. 2. Seafood— Preservation—Handbooks, manuals, etc. 3. Cold storage—Planning—Handbooks, manuals, etc. 4. Fishery management—Handbooks, manuals, etc. 5. Refrigeration and refrigeration machinery—Handbooks, manuals, etc. 6. Frozen fishery products—Handbooks, manuals, etc. I. Title. II. Kramer, Donald E. III. Series: Alaska Sea Grant College Program ; MAB-60. SH336.F7 K65 2007 ISBN 1-56612-119-1 Credits The work for this book was funded in part by the NOAA Office of Sea Grant, U.S. Department of Commerce, under grants NA76RG0476 (OSU), NA86RG0050 (UAF), and NA76RG0119 (UW); projects A/ESG-3 (OSU), A/151-01 (UAF), and A/FP-7 (UW), and by appropriations made by the Oregon, Alaska, and Washington state legislatures. Publishing is supported by grant NA06OAR4170013, project A/161-01. Sea Grant is a unique partnership with public and private sectors, combining research, education, and technology transfer for public service. This national network of universities meets the changing environmental and economic needs of people in our coastal, ocean, and Great Lakes regions. -

Brochure-Product-Range.Pdf
PRODUCT RANGE 2015 edition ANSI Standard 60 NSF® CERTIFIED HALAL M ISLAMIC FOOD AND NUTRITION ® COUNCIL OF AMERICA Rue Joseph Wauters, 144 ISO 9001:2008 (Quality) / OHSAS 18001:2007 (Health/ B-4480 Engis Safety) / ISO 14001:2004 (Environment) / ISO 22000:2005 www.globulebleu.com (Food Safety) / FSSC 22000:2013 (Food Safety). Tel. +32 (0) 4 273 93 58 Our food grade phosphates are allergen free, GMO free, Fax. +32 (0) 4 275 68 36 BSE/TSE free. www.prayon.com mail. [email protected] Design by www.prayon.com PRODUCT RANGE | 11 TABLE OF CONTENTS HORTICULTURE APPLICATIONS HORTIPRAY® RANGE FOR HORTICULTURE* FOOD AND INDUSTRIAL APPLICATIONS PRODUCT NAME Bulk density P O pH N-NH Made 2 5 4 MONOAMMONIUM PHOSPHATE - NH4H2PO4 in 3 3 % 1% % Sodium orthophosphates ................................................................................... 03 g/cm lbs/ft indicative indicative indicative Water-soluble fertilisers. Sodium pyrophosphates .................................................................................... 04 HORTIPRAY® MAP Horticultural Grade 0.9 56 61 4.5 12 Sodium tripolyphosphates ................................................................................. 05 HORTIPRAY® MAP 12.60 Horticultural Grade 0.9 56 60 5 12.1 Water-soluble fertilisers; Sodium polyphosphates ..................................................................................... 06 HORTIPRAY® MAP anticalc Horticultural Grade 0.9 56 61 4.5 12 preventive action against clogging. Potassium orthophosphates ............................................................................. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

Phosphates' Function in Poultry Products
[Salt/Sodium Reduction] Vol. 17 No. 8 August 2007 ww Phosphates’ Function in Poultry Products By Andrew G. Ebert, Ph.D., Contributing Editor Food-grade phosphates perform a number of functions in processed poultry products. These properties include raising pH, increasing ionic strength and reacting with protein, which improves the water-holding capacity of the functional myofibrillar protein in poultry, thereby decreasing cook-cool loss, and creating improved and uniform yields. Phosphates protect against oxidation of lipids by binding metal ions that accelerate oxidation. They protect and preserve the natural flavor and color of poultry, and they solubilize protein, which is required in producing formed and restructured products, such as delicatessen rolls and chicken nuggets. Additive effects Studies with poultry products began over 40 years ago. Early applications involved static holding in solutions containing sodium tripolyphosphate (STPP), an alkaline phosphate, and sodium hexametaphosphate (SHMP). The most-notable food-technological effects were a 6.2% increase in moisture content and a rise in pH from 6.1 to 6.6, due to the addition of phosphate. With levels of phosphates used today, a more-modest rise (0.1 to 0.3 pH units, depending upon the phosphate or blend) would be anticipated. More recent studies have shown that the use of phosphates increases the temperature required for denaturation of protein and, thus, reduces cook-cool loss, leading to an end product with greater succulence. The addition of STPP and sodium chloride (NaCl) in poultry-processing formulations has been shown to have a number of advantages. First, STPP and NaCl increase ionic strength and synergistically extract myofibrillar protein. -

Use of Thin Layer Chromatography in Detecting the Addition and Degradation of Sodium Phosphates in Seafood Products
USE OF THIN LAYER CHROMATOGRAPHY IN DETECTING THE ADDITION AND DEGRADATION OF SODIUM PHOSPHATES IN SEAFOOD PRODUCTS By PHILLIP BLAINE HOLMBERG A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2010 1 © 2010 Phillip Blaine Holmberg 2 To my parents, Eric and Helen Holmberg, and to all who nurtured my intellectual curiosity, academic interests, and sense of scholarship throughout my lifetime, making this milestone possible 3 ACKNOWLEDGMENTS I would like to thank my major advisor, Dr. Otwell, for all of his advice, support and opportunities to gain knowledge and experience. I would also like to thank my other graduate committee members, Dr. Marty Marshall and Dr. Frank Chapman. I extend a special thanks to Sven-Eric Ebeling and everyone at Chemische Fabrik Budenheim KG for their teachings and generous hospitality during my research in Germany. I thank Laura Garrido for helping me with everything along the way. I also thank Victor Garrido and all of my lab mates, past and present, Steven D’uva, Alex Fortunado, Marcus Ladd, Zach Lowenstein, Kelley Zhou, and Ivan Lozano. I thank Zina Williams for all of her help and support. Finally, I would like to thank my friends and family for all the good times and always being there for me. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS .................................................................................................. 4 LIST OF TABLES ........................................................................................................... -

QUALITY CHOICE SINGLE SALINE ENEMA LAXATIVE Drug Facts
QUALITY CHOICE SINGLE SALINE LAXATIVE- sodium phosphate, dibasic and sodium phosphate, monobasic, unspecified form enema CDMA Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- QUALITY CHOICE SINGLE SALINE ENEMA LAXATIVE Drug Facts Active ingredients (in each 118mL Purpose delivered dose) Dibasic Sodium Phosphate 7 g Saline laxative Monobasic Sodium Phosphate 19 g Saline laxative Use Relieves occasional constipation Warnings For rectal use only. Dosage warning Using more than one enema in 24 hours can be harmful. Ask a doctor before use if you already used a laxative for more than 1 week have kidney disease have heart problems are dehydrated are 55 years old or older are on a sodium-restricted diet Ask a doctor before using any laxative if you have abdominal pain, nausea, or vomiting a sudden change in bowel habits lasting more than 2 weeks already used a laxative for more than 1 week When using this product do not use more than directed. Serious side effects may occur from excess dosage do not use for more than 3 days Stop use and ask a doctor if you have bleeding the condtion worsens or does not improve within 7 days you have no bowel movement within 30 minutes of enema use you have symptoms of dehydration (thirstiness, dizziness, vomiting, urinating less often than normal) These symptoms may indicate a serious condition. If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. -

Federal Register/Vol. 63, No. 98/Thursday, May 21, 1998/Rules and Regulations
27836 Federal Register / Vol. 63, No. 98 / Thursday, May 21, 1998 / Rules and Regulations concludes that the proposed use of the (address above) between 9 a.m. and 4 document. Any objections received in additive is safe, that the additive will p.m., Monday through Friday. response to the regulation may be seen achieve its intended technical effect, Any person who will be adversely in the Dockets Management Branch and therefore, that the regulations in affected by this regulation may at any between 9 a.m. and 4 p.m., Monday § 178.2010 should be amended as set time on or before June 22, 1998, file through Friday. forth below. with the Dockets Management Branch List of Subjects in 21 CFR Part 178 In accordance with § 171.1(h) (21 CFR (address above) written objections thereto. Each objection shall be Food additives, Food packaging. 171.1(h)), the petition and the Therefore, under the Federal Food, documents that FDA considered and separately numbered, and each numbered objection shall specify with Drug, and Cosmetic Act and under relied upon in reaching its decision to authority delegated to the Commissioner approve the petition are available for particularity the provisions of the regulation to which objection is made of Food and Drugs and redelegated to inspection at the Center for Food Safety the Director, Center for Food Safety and and Applied Nutrition by appointment and the grounds for the objection. Each numbered objection on which a hearing Applied Nutrition, 21 CFR part 178 is with the information contact person amended as follows: listed above. -

Vipel® Chemical Resistance Guide
Vipel® Chemical Resistance Guide CORROSION RESISTANT RESINS FOR THE HARSHEST ENVIRONMENTS The Right Chemistry for the Harshest Environments – AOC is leading the composites industry in the fight against damaging and costly corrosion. Our Vipel corrosion-resistant resins set the global standard for proven performance in a wide range of fibre-reinforced polymer (FRP) applications, including pipes, tanks, water and wastewater treatment, chemical processing, pulp and paper, mining and power generation. With unparalleled strength and durability, AOC resins combat corrosion in even the harshest conditions. 2 INTRODUCTION TO CORROSION RESISTANT RESINS Selecting a Resin System • Length of exposure to the medium if less than continuous. For example, the laminate may need to This guide is designed to assist fabricators of FRP withstand only occasional splashes of exposure. components in selecting the appropriate resin for parts which will be exposed to highly corrosive environments. • Process description, where a reaction such as The data presented is the result of years of extensive neutralization takes place in the tank. laboratory testing and actual field exposure in North • Fire retardancy, where applicable, including flame America and Europe. spread rating and smoke requirements. Resin system selection is determined by the chemical service and environment to which the equipment will Sorting Out the Details be exposed, end-user specifications and preferences, Normally a suitable resin can be selected from the or fabricator recommendation. AOC Corrosion advisors Chemical Resistance Guide based on the information in also are available any time to answer questions. the “Gathering Data” section above. A few comments on information featured in this guide should be noted: Users frequently specify the resin system and laminate construction for particular applications. -
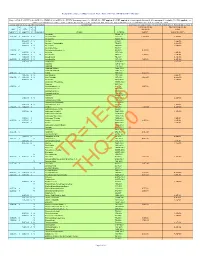
Composite Worker Air RSL May 2021 HQ10
Regional Screening Level (RSL) Composite Worker Ambient Air Table (TR=1E-06, HQ=1) May 2021 Key: I = IRIS; P = PPRTV; O = OPP; A = ATSDR; C = Cal EPA; X = PPRTV Screening Level; H = HEAST; W = TEF applied; E = RPF applied; G = user's guide Section 5; M = mutagen; V = volatile; R = RBA applied ; c = cancer; n = noncancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; SSL values are based on DAF=1; m = ceiling limit exceeded; s = Csat exceeded. Toxicity and Chemical-specific Information Contaminant Carcinogenic Target Risk (TR) = 1E-06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1E-06 THI=1 (ug/m3)-1 y (mg/m3) y l mutagen Analyte CAS No. (ug/m3) (ug or fibers/m3) Acephate 30560-19-1 2.2E-06 I 9.0E-03 I V Acetaldehyde 75-07-0 5.6E+00 3.9E+01 Acetochlor 34256-82-1 3.1E+01 A V Acetone 67-64-1 1.4E+05 2.0E-03 X Acetone Cyanohydrin 75-86-5 8.8E+00 6.0E-02 I V Acetonitrile 75-05-8 2.6E+02 V Acetophenone 98-86-2 1.3E-03 C Acetylaminofluorene, 2- 53-96-3 9.4E-03 2.0E-05 I V Acrolein 107-02-8 8.8E-02 1.0E-04 I 6.0E-03 I M Acrylamide 79-06-1 1.2E-01 2.6E+01 1.0E-03 I V Acrylic Acid 79-10-7 4.4E+00 6.8E-05 I 2.0E-03 I V Acrylonitrile 107-13-1 1.8E-01 8.8E+00 6.0E-03 P Adiponitrile 111-69-3 2.6E+01 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb Sulfone 1646-88-4 Aldicarb sulfoxide 1646-87-3 4.9E-03 I V Aldrin 309-00-2 2.5E-03 1.0E-04 X V Allyl Alcohol 107-18-6 4.4E-01 6.0E-06 C 1.0E-03 I V Allyl Chloride 107-05-1 2.0E+00 4.4E+00 5.0E-03 P Aluminum 7429-90-5 2.2E+01 Aluminum Phosphide 20859-73-8 -
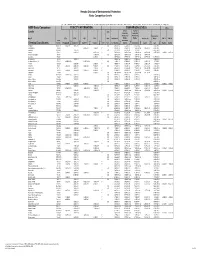
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V