Survey of Fertilizers and Related Materials for Perchlorate (Clo4 )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Material Safety Data Sheet
MATERIAL SAFETY DATA SHEET SRM Supplier: National Institute of Standards and Technology SRM Number: 186g Standard Reference Materials Program MSDS Number: 186g 100 Bureau Drive, Stop 2321 SRM Name: pH Standards Gaithersburg, Maryland 20899-2321 Potassium Dihydrogen Phosphate (186-I-g); Disodium Hydrogen Phosphate (186-II-g) Date of Issue: 07 November 2003 MSDS Coordinator: Carmen Davis Phone: (301) 975-6776 FAX: (301) 926-4751 ChemTrec: 1-800-424-9300 E-mail: [email protected] SECTION I. MATERIAL IDENTIFICATION Material Name: pH Standards: Potassium Dihydrogen Phosphate (186-I-g); Disodium Hydrogen Phosphate (186-II-g) Description: SRM 186g consists of two components, each prepared to ensure high purity and uniformity: KH2PO4, Potassium Dihydrogen Phosphate (186-I-g) and Na2HPO4, Disodium Hydrogen Phosphate (186-II-g). However, neither SRM component is certified for purity of substance. A unit of SRM 186g consists of 30 g of potassium dihydrogen phosphate (186-I-g) and 45 g of disodium hydrogen phosphate (186-II-g), each contained in its respective clear glass bottle. Other Designations: Potassium Dihydrogen Phosphate (potassium acid phosphate; monopotassium phosphate; potassium diphosphate; potassium biphosphate; potassium orthophosphate; potassium dihydrogen phosphate) Disodium Hydrogen Phosphate (disodium phosphate; disodium acid orthophosphate; soda phosphate; disodium phosphoric acid; disodium monohydrogen phosphate; monohydrogen disodium phosphate; DSP; sodium phosphate; sodium phosphate (Na2HPO4); hydrogen disodium phosphate; phosphoric acid, disodium salt; sodium monohydrogen phosphate; anhydrous sodium acid phosphate; disodium acid phosphate; dibasic sodium phosphate; disodium orthophosphate; disodium hydrogenorthophosphate) Name Chemical Formula CAS Registry Number Potassium Dihydrogen Phosphate KH2PO4 7778-77-0 Disodium Hydrogen Phosphate Na2HPO4 7558-79-4 DOT Classification: Potassium dihydrogen phosphate and disodium hydrogen phosphate are not regulated by DOT. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Vaccines Chart
VACCINE CONTAINS BREAKDOWN Adenovirus human-diploid Human-diploid fibroblast cell cultures (strain WI-38) is a diploid human cell strain composed of fibroblasts derived ***Vaccine fibroblast cell from lung tissue of a 3-months gestation aborted female fetus (https://en.wikipedia.org/wiki/WI-38) indicated for cultures (strain WI- active 38), Dulbecco’s 2) Dulbecco’s Modified Eagle’s Medium is the mostly broadly suitable medium for many adherent cell phenotypes immunizatio Modified Eagle’s among defined media for cell and tissue culture. The Dulbecco’s modification is an enhanced supplementary n for the Medium, fetal formulation that boosts select amino acid and vitamin content of the original Eagle’s medium is up to fourfold. Our prevention bovine serum, selection includes a range of glucose concentrations, as well as formulations with and without L-glutamine. Products of febrile sodium bicarbonate, without the pH indicator phenol red are available for estrogen-sensitive applications, and our comprehensive offering acute monosodium includes convenient, ready-to-use liquid formats, as well as economical powdered media for easier storage and longer respiratory glutamate, sucrose, shelf life (https://www.sigmaaldrich.com/life-science/cell-culture/classical-media-salts/dmem.html) disease D-mannose, caused by Fructose, dextrose, 3) Fetal bovine serum is the liquid fraction of clotted blood from fetal calves, depleted of cells, fibrin and clotting Adenovirus human serum factors, but containing a large number of nutritional and macromolecular factors essential for cell growth. Bovine Type 4 and albumin, potassium serum albumin is the major component of FBS. Growth factors in FBS are essential for the maintenance and growth of Type 7. -

Thirty-Fourth List of New Mineral Names
MINERALOGICAL MAGAZINE, DECEMBER 1986, VOL. 50, PP. 741-61 Thirty-fourth list of new mineral names E. E. FEJER Department of Mineralogy, British Museum (Natural History), Cromwell Road, London SW7 5BD THE present list contains 181 entries. Of these 148 are Alacranite. V. I. Popova, V. A. Popov, A. Clark, valid species, most of which have been approved by the V. O. Polyakov, and S. E. Borisovskii, 1986. Zap. IMA Commission on New Minerals and Mineral Names, 115, 360. First found at Alacran, Pampa Larga, 17 are misspellings or erroneous transliterations, 9 are Chile by A. H. Clark in 1970 (rejected by IMA names published without IMA approval, 4 are variety because of insufficient data), then in 1980 at the names, 2 are spelling corrections, and one is a name applied to gem material. As in previous lists, contractions caldera of Uzon volcano, Kamchatka, USSR, as are used for the names of frequently cited journals and yellowish orange equant crystals up to 0.5 ram, other publications are abbreviated in italic. sometimes flattened on {100} with {100}, {111}, {ill}, and {110} faces, adamantine to greasy Abhurite. J. J. Matzko, H. T. Evans Jr., M. E. Mrose, lustre, poor {100} cleavage, brittle, H 1 Mono- and P. Aruscavage, 1985. C.M. 23, 233. At a clinic, P2/c, a 9.89(2), b 9.73(2), c 9.13(1) A, depth c.35 m, in an arm of the Red Sea, known as fl 101.84(5) ~ Z = 2; Dobs. 3.43(5), D~alr 3.43; Sharm Abhur, c.30 km north of Jiddah, Saudi reflectances and microhardness given. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Bojarite, Found and Characterized by a Research Team Led by Nikita Chukanov (Russian Academy of Sciences, Moscow)
For 2020 the “Mineral of the Year” award has been assigned to bojarite, found and characterized by a research team led by Nikita Chukanov (Russian Academy of Sciences, Moscow). The full description of the new mineral is available here: Chukanov, N.V., Möhn, G., Zubkova, N.V., Ksenofontov, D.A., Pekov, I.V., Agakhanov, A.A., Britvin, S.N., Desor, J. (2020): Bojarite, Cu3(N3C2H2)3(OH)Cl2·6H2O, a new mineral species with a microporous metal-organic framework from the guano deposit at Pabellón de Pica, Iquique Province, Chile. Mineralogical Magazine, 84, 921-927. Bojarite was discovered in a guano deposit on the northern slope of the Pabellón de Pica Mountain, 1.5 km south of Chanabaya village, Iquique Province, Tarapacá Region, Chile. The mineral occurs as blue fine-grained porous aggregates a few mm wide. Associated minerals are salammoniac, halite, chanabayaite, nitratine, and belloite (Fig. 1). Its ideal chemical formula is Cu3(N3C2H2)3(OH)[Cl2(H2O)4]·2H2O, hence bojarite is a copper triazolate mineral. Bojarite crystallizes in the cubic system, and has space group Fd 3c, with a = 24.8047(5) Å. The crystal structure of bojarite has been refined by the Rietveld method and is definitely elegant: three Cu2+ cations are linked by an hydroxyl anion at the center of an equilateral triangle and are also 2+ connected to two nitrogen atoms of the triazole ring, leading to the formation of [Cu3(trz)3(OH)] – building blocks [where trz = 1,2,4-triazole anion (N3C2H2) ]. The third nitrogen atom of the triazole ring connects the triangular unit with adjacent units, giving rise to a three-dimensional network. -

Italian Type Minerals / Marco E
THE AUTHORS This book describes one by one all the 264 mi- neral species first discovered in Italy, from 1546 Marco E. Ciriotti was born in Calosso (Asti) in 1945. up to the end of 2008. Moreover, 28 minerals He is an amateur mineralogist-crystallographer, a discovered elsewhere and named after Italian “grouper”, and a systematic collector. He gradua- individuals and institutions are included in a pa- ted in Natural Sciences but pursued his career in the rallel section. Both chapters are alphabetically industrial business until 2000 when, being General TALIAN YPE INERALS I T M arranged. The two catalogues are preceded by Manager, he retired. Then time had come to finally devote himself to his a short presentation which includes some bits of main interest and passion: mineral collecting and information about how the volume is organized related studies. He was the promoter and is now the and subdivided, besides providing some other President of the AMI (Italian Micromineralogical As- more general news. For each mineral all basic sociation), Associate Editor of Micro (the AMI maga- data (chemical formula, space group symmetry, zine), and fellow of many organizations and mine- type locality, general appearance of the species, ralogical associations. He is the author of papers on main geologic occurrences, curiosities, referen- topological, structural and general mineralogy, and of a mineral classification. He was awarded the “Mi- ces, etc.) are included in a full page, together cromounters’ Hall of Fame” 2008 prize. Etymology, with one or more high quality colour photogra- geoanthropology, music, and modern ballet are his phs from both private and museum collections, other keen interests. -

List of Designated Additives
The Japan Food chemical Research Faundation List of Designated Additives The substances below are the designated additives appearing in Table 1, as mentioned in Article 12 of the Enforcement Regulations under the Food Sanitation Law. These additives are listed here in alphabetic order. They are 455 in total as of July 03, 2018. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese table. 16 Acesulfame Potassium(Acesulfame K) 20 Acetaldehyde 322 Acetic Acid, Glacial 23 Acetone 22 Acetophenone 17 Acetylated Distarch Adipate 19 Acetylated Distarch Phosphate 18 Acetylated Oxidized Starch 5 Adipic Acid 26 Advantame 32 DL-Alanine 190 Aliphatic Higher Alcohols 191 Aliphatic Higher Aldehydes (except those generally recognized as highly toxic) 192 Aliphatic Higher Hydrocarbons (except those generally recognized as highly toxic) 178 Allyl Cyclohexylpropionate 364 Allyl Hexanoate (Allyl Caproate) 53 Allyl Isothiocyanate (Volatile Oil of Mustard) 429 Aluminum Ammonium Sulfate (Crystal: Ammonium Alum, Desiccated: Burnt Ammonium Alum) 430 Aluminum Potassium Sulfate (Crystal: Alum or Potassium Alum, Desiccated: Burnt Alum) 29 (3-Amino-3-carboxypropyl)dimethylsulfonium chloride 43 Ammonia 35 Ammonium Alginate 240 Ammonium Bicarbonate (Ammonium Hydrogen Carbonate) 237 Ammonium Carbonate 81 Ammonium Chloride 446 Ammonium Dihydrogen Phosphate (Ammonium Phosphate, Monobasic or Monoammonium Phosphate) 44 Ammonium isovalerate 98 Ammonium Persulfate 431 Ammonium Sulfate 30 Amylalcohol -

Toxicological Profile for Nitrate and Nitrite
NITRATE AND NITRITE 151 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Information regarding the chemical identity of nitrate and nitrite is provided in Table 4-1 and information regarding the chemical identity of selected inorganic nitrate and nitrite compounds is provided in Table 4-2. Information regarding ammonia and urea is provided in Table 4-3. Inorganic nitrate and nitrite are naturally occurring ionic species that are part of the earth’s nitrogen cycle (see Figure 5-1). These anions are the products formed via the fixation of nitrogen and oxygen. Chemical processes, biological processes, and microbial processes in the environment convert nitrogen compounds to nitrite and nitrate via nitrogen fixation and nitrification. Compounds such as urea are converted via hydrolysis to ammonia, protonation of ammonia to ammonium (cation), followed by oxidation of ammonium to form nitrite, and then oxidation to form nitrate. Nitrate and nitrite are not neutral compounds, but rather the ionic (anionic; negatively charged) portions of compounds, commonly found in commerce as organic and inorganic salts. As used in this profile, the word “ion” is implied and not used, unless added for clarity. Nitrate and nitrite typically exist in the environment as highly water-soluble inorganic salts, often bound when not solubilized to metal cations such as sodium or potassium. The nitrate ion is the more stable form as it is chemically unreactive in aqueous solution; however, it may be reduced through biotic processes with nitrate reductase to the nitrite ion. The nitrite ion is readily oxidized back to the nitrate ion via Nitrobacter (a genus of proteobacteria), or conversely, the nitrite ion may be reduced to various compounds (IARC 2010; WHO 2011b). -

Niter KNO3 C 2001-2005 Mineral Data Publishing, Version 1
Niter KNO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. Acicular crystals, in silky tufted to branching aggregates, may be flowerlike, to 2 cm; commonly in thin crusts, granular, earthy, mealy, massive. Twinning: Common on {110}, forming pseudohexagonal trillings. Physical Properties: Cleavage: {011}, nearly perfect; {010}, good; {110}, imperfect. Fracture: Subconchoidal to uneven. Tenacity: Brittle. Hardness = 2 D(meas.) = 2.109(2) D(calc.) = 2.104 Soluble in H2O, taste saline and cooling. Optical Properties: Transparent. Color: Colorless to white, may be gray from impurities. Streak: White. Luster: Vitreous. Optical Class: Biaxial (–). Orientation: X = c; Y = a; Z = b. Dispersion: r< v,weak. α = 1.332 β = 1.504 γ = 1.504 2V(meas.) = 7◦ Cell Data: Space Group: P mcn. a = 5.414 b = 9.164 c = 6.431 Z = 4 X-ray Powder Pattern: Synthetic. 3.78 (100), 3.73 (56), 3.033 (55), 2.647 (55), 2.661 (41), 2.192 (41), 2.763 (28) Chemistry: (1) Identified by correspondence of optical data and X-ray powder pattern with that of synthetic material. Occurrence: In some caves, typically formed by bacterial action on animal matter, as bat guano, or from vegetable matter, such as humus, exposed to seeping groundwater; an efflorescence on soils or cliff faces in arid regions. Association: Nitratine, nitrocalcite, nitromagnesite, epsomite, gypsum, calcite. Distribution: Usually in minor amounts, but may form substantial masses, into the thousands of tons, especially in some cave deposits. Typical large-scale occurrences are represented by: Pulo di Molfetta Cave, Apulia, Italy. -
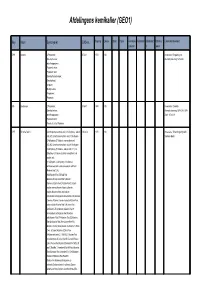
Afdelingens Kemikalier (GEO1)
Afdelingens kemikalier (GEO1) Key Navn Synonymer CAS-nr. Bygning Lokale Skab Hylde Kemikalie Købsdato Udløbsdat Batchnu Lokale kommentarer: nummer o mmer 7299 Acetone 2-Propanone; 67-64-1 1674 138 Anvendelse: Rengøring mm. Dimethyl ketone; Alternativ placering: 1672-242 beta-Ketopropane; Pyroacetic ether; Pyroacetic acid; Dimethylformaldehyde; Dimethylketal; propane; Methyl ketone; Propanone; Propanon; 535 Acetone p.a. 2-Propanone; 67-64-1 1674 138 Anvendelse: Cleanlab Dimethyl ketone; Alternativ placering: 1674-234; 1674- beta-Ketopropane; 238A; 1674-218 Pyroaceticether; Pyroacetic ether;Propanon; 1797 Alizarine Red S 2-Anthraquinonesulfonic acid, 3,4-dihydroxy-, sodium 130-22-3 1674 138 Anvendelse: Til farvning af tyndslib salt (6CI);2-Anthracenesulfonic acid, 9,10-dihydro- Stephane Bodin 3,4-dihydroxy-9,10-dioxo-, monosodium salt (8CI,9CI);2-Anthracenesulfonic acid, 9,10-dihydro- 3,4-dihydroxy-9,10-dioxo-, sodium salt (1:1);3,4- Dihydroxy-9,10-dioxo-2-anthracenesulfonic acid sodium salt; 9,10-Dihydro-3,4-dihydroxy-9,10-dioxo-2- anthracenesulfonic acid monosodium salt;Acid Mordant Red S 80; Acid Mordant Red SW;Acid Red Alizarine;Ahcoquinone Red S;Alizarin Carmine;Alizarin Red C;Alizarin Red S;Alizarin sodium monosulfonate;Alizarin sulfonate sodium;Alizarinsulfonic acid sodium salt;Alizarinsulfonsyrenatriumsalt;Alizarin S;Alizarine Carmine;Alizarine Carmine Indicator;Alizarin Red, water soluble;Alizarine Red A;Alizarine Red AS;Alizarine Red Indicator;Alizarine Red S monosodium salt;Alizarine Red S sodium salt;Alizarine Red SW;Alizarine Red SZ;Alizarine Red W;Alizarine Red WA;Alizarine Red WS; Alizarine Red for Wool;Alizarine S;Alizarine S Extra Conc. A Export;Alizarine S Extra Pure A;Alizarinsulfonate;C.I. -

The Thermodynamics of Selenium Minerals in Near-Surface Environments
minerals Review The Thermodynamics of Selenium Minerals in Near-Surface Environments Vladimir G. Krivovichev 1,*, Marina V. Charykova 2 ID and Andrey V. Vishnevsky 2 1 Department of Mineralogy, Institute of Earth Sciences, St. Petersburg State University, 7/9 University Embankment, Saint Petersburg 199034, Russia 2 Department of Geochemistry, Institute of Earth Sciences, St. Petersburg State University, 7/9 University Embankment, Saint Petersburg 199034, Russia; [email protected] (M.V.C.); [email protected] (A.V.V.) * Correspondence: [email protected]; Tel.: +7-812-328-9481 Received: 18 August 2017; Accepted: 4 October 2017; Published: 6 October 2017 Abstract: Selenium compounds are relatively rare as minerals; there are presently only 118 known mineral species. This work is intended to codify and systematize the data of mineral systems and the thermodynamics of selenium minerals, which are unstable (selenides) or formed in near-surface environments (selenites), where the behavior of selenium is controlled by variations of the redox potential and the acidity of solutions at low temperatures and pressures. These parameters determine the migration of selenium and its precipitation as various solid phases. All selenium minerals are divided into four groups—native selenium, oxide, selenides, and oxysalts—anhydrous selenites (I) and hydrous selenites and selenates (II). Within each of the groups, minerals are codified according to the minimum number of independent elements necessary to define the composition of the mineral system. Eh–pH diagrams were calculated and plotted using the Geochemist’s Workbench (GMB 9.0) software package. The Eh–pH diagrams of the Me–Se–H2O systems (where Me = Co, Ni, Fe, Cu, Pb, Zn, Cd, Hg, Ag, Bi, As, Sb, Al and Ca) were plotted for the average contents of these elements in acidic waters in the oxidation zones of sulfide deposits.