Toxicological Profile for Nitrate and Nitrite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parental Brine Evolution in the Chilean Nitrate Deposits (Pedro De Valdivia, II Region De Antofagasta) : Mineralogical and Petro
Third ISAG, S1 Malo (France), 17-19/9/1996 707 PARENTAL BRINE EVOLUTION IN THE CHILEAN NITRATE DEPOSITS (Pedro de Valdivia, I1 Regi6n de Antofagasta). MINERALOGICAL AND PETROGRAPHIC DATA Marta VEGA(l), G. CHONG(') and Juan J. PUEy0t2). ('1 Departamento de Ciencias Geol6gicas. Universidad Cat6lica del Norte. Avda.Angamos, 0610. Casilla 1280. Antofagasta. (2) Facultat de Geologia. Universitat de Barcelona. Zona Universitaria de Pedralbes. 08028 Barcelona . KEYWORDS: nitrates, iodates, saline minerals, paragenesis, porosity, brine evolution. INTRODUCTION The Chilean nitrate deposits have been formed by complex paragenesis of saline minerals (locally named caliche) that infill the porosity of rocks ranging in age from Paleozoic to Cenozoic. Minerals which are normally extremely rare - nitrates, nitrate-sulphates, iodates and iodate-sulphates - are common in the Chilean nitrates. Located in the Atacama Desert (North Chile; between 19'30' and 2.5'30' S latitude, and 69'30' and 70'30' W longitude), the deposits follow an irregular N-S swathe, a few km wide (reaching a maximum of several tens of km). The distribution of the deposits parallels the contact between the Coastal Range and the Central Depression. The nitrate (and saline) ores can infill either cracks (joints, fractures) in the country rocks (volcanic, intrusive or sedimentary), porosity in breccias and conglomerates from alluvial fans and pediments, or porosity created by previous alteration processes. Ericksen (1981) divided the different styles of occurrence as deposits in rock and sedimentary deposits. Deposits in rock are characterized by Old alluvial sediments Fig. 1. Distribution of worked nitrate areas in the Pedro de Valdivia area. See the relationships between the nitrate deposits and tertiary alluvial sediments on the NW alluvial fans. -

Brief Guide to the Nomenclature of Organic Chemistry
1 Brief Guide to the Nomenclature of Table 1: Components of the substitutive name Organic Chemistry (4S,5E)-4,6-dichlorohept-5-en-2-one for K.-H. Hellwich (Germany), R. M. Hartshorn (New Zealand), CH3 Cl O A. Yerin (Russia), T. Damhus (Denmark), A. T. Hutton (South 4 2 Africa). E-mail: [email protected] Sponsoring body: Cl 6 CH 5 3 IUPAC Division of Chemical Nomenclature and Structure suffix for principal hept(a) parent (heptane) one Representation. characteristic group en(e) unsaturation ending chloro substituent prefix 1 INTRODUCTION di multiplicative prefix S E stereodescriptors CHEMISTRY The universal adoption of an agreed nomenclature is a key tool for 2 4 5 6 locants ( ) enclosing marks efficient communication in the chemical sciences, in industry and Multiplicative prefixes (Table 2) are used when more than one for regulations associated with import/export or health and safety. fragment of a particular kind is present in a structure. Which kind of REPRESENTATION The International Union of Pure and Applied Chemistry (IUPAC) multiplicative prefix is used depends on the complexity of the provides recommendations on many aspects of nomenclature.1 The APPLIED corresponding fragment – e.g. trichloro, but tris(chloromethyl). basics of organic nomenclature are summarized here, and there are companion documents on the nomenclature of inorganic2 and Table 2: Multiplicative prefixes for simple/complicated entities polymer3 chemistry, with hyperlinks to original documents. An No. Simple Complicated No. Simple Complicated AND overall -

Genetic Basis for Nitrate Resistance in Desulfovibrio Strains
ORIGINAL RESEARCH ARTICLE published: 21 April 2014 doi: 10.3389/fmicb.2014.00153 Genetic basis for nitrate resistance in Desulfovibrio strains Hannah L. Korte 1,2,SamuelR.Fels2,3, Geoff A. Christensen 1,2,MorganN.Price2,4, Jennifer V. Kuehl 2,4, Grant M. Zane 1,2, Adam M. Deutschbauer 2,4,AdamP.Arkin2,4 and Judy D. Wall 1,2,3* 1 Department of Biochemistry, University of Missouri, Columbia, MO, USA 2 Ecosystems and Networks Integrated with Genes and Molecular Assemblies, Berkeley, CA, USA 3 Department of Molecular Microbiology and Immunology, University of Missouri, Columbia, MO, USA 4 Physical Biosciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA Edited by: Nitrate is an inhibitor of sulfate-reducing bacteria (SRB). In petroleum production sites, Hans Karl Carlson, University of amendments of nitrate and nitrite are used to prevent SRB production of sulfide that California, Berkeley, USA causes souring of oil wells. A better understanding of nitrate stress responses in Reviewed by: the model SRB, Desulfovibrio vulgaris Hildenborough and Desulfovibrio alaskensis G20, Dimitry Y. Sorokin, Delft University of Technology, Netherlands will strengthen predictions of environmental outcomes of nitrate application. Nitrate Wolfgang Buckel, inhibition of SRB has historically been considered to result from the generation of small Philipps-Universität Marburg, amounts of nitrite, to which SRB are quite sensitive. Here we explored the possibility Germany that nitrate might inhibit SRB by a mechanism other than through nitrite inhibition. *Correspondence: We found that nitrate-stressed D. vulgaris cultures grown in lactate-sulfate conditions Judy D. Wall, Department of Biochemistry, University of eventually grew in the presence of high concentrations of nitrate, and their resistance Missouri, 117 Schweitzer Hall, continued through several subcultures. -

Safety Data Sheet
SAFETY DATA SHEET According to JIS Z 7253:2019 Revision Date 14-Jan-2021 Version 1.03 Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name Potassium Nitrite Product code 165-27322,169-27325 Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-5964 Supplier FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome, Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-2029 Emergency telephone number +81-6-6203-3741 / +81-3-3270-8571 Recommended uses and For research use only restrictions on use Section 2: HAZARDS IDENTIFICATION GHS classification Classification of the substance or mixture Oxidizing solids Category 2 Reproductive Toxicity Category 2 (additional) Specific target organ toxicity (single exposure) Category 1 Category 1 blood Short-term (acute) hazardous to the aquatic environment Category 2 Long-term (chronic) hazardous to the aquatic environment Category 2 Pictograms Signal word Danger Hazard statements H272 - May intensify fire; oxidizer H361 - Suspected of damaging fertility or the unborn child H362 - May cause harm to breast-fed children H401 - Toxic to aquatic life H411 - Toxic to aquatic life with long lasting effects H370 - Causes damage to the following organs: blood Precautionary statements-(Prevention) • Keep away from heat/sparks/open flames/hot surfaces. — No smoking • Keep/Store away from clothing/combustible materials • Obtain special instructions before use • Do not handle until all safety precautions -

Potassium Nitrate
Common Name: POTASSIUM NITRATE CAS Number: 7757-79-1 RTK Substance number: 1574 DOT Number: UN 1486 Date: March 1998 Revision: November 2004 --------------------------------------------------------------------------- --------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Potassium Nitrate can affect you when breathed in. No occupational exposure limits have been established for * Contact can cause eye and skin irritation. Potassium Nitrate. This does not mean that this substance is * Breathing Potassium Nitrate can irritate the nose and not harmful. Safe work practices should always be followed. throat causing sneezing and coughing. * High levels can interfere with the ability of the blood to WAYS OF REDUCING EXPOSURE carry Oxygen causing headache, fatigue, dizziness, and a * Where possible, enclose operations and use local exhaust blue color to the skin and lips (methemoglobinemia). ventilation at the site of chemical release. If local exhaust Higher levels can cause trouble breathing, collapse and ventilation or enclosure is not used, respirators should be even death. worn. * Potassium Nitrate may affect the kidneys and cause * Wear protective work clothing. anemia. * Wash thoroughly immediately after exposure to Potassium Nitrate. IDENTIFICATION * Post hazard and warning information in the work area. In Potassium Nitrate is a transparent, white or colorless, addition, as part of an ongoing education and training crystalline (sand-like) powder or solid with a sharp, salty effort, communicate all information on the health and taste. It is used to make explosives, matches, fertilizer, safety hazards of Potassium Nitrate to potentially fireworks, glass and rocket fuel. exposed workers. REASON FOR CITATION * Potassium Nitrate is on the Hazardous Substance List because it is cited by DOT. * Definitions are provided on page 5. -

Sodium Nitrate 2005
CONTENTS 1 Added to the Natural List b) Prof. 0.Van Clamput, UnksWtGent c) 07. T.K. Harts, Univ. of Wbrnia - Davis aer, Nrrt. AgrScuituml 0 W.Vooqt,Werg~ l3emamh cmw Reply to the 2004 IFOAM Evaluation of Natuml Sodium Nitrate 'IFOAM 2004" NORTH AMERICA February 25,2005 Mr. Robert Pooler Agricultural Marketing Specialist USDAIAMS/TM/NOP Room 25 10-So., Ag Stop 0268 P.O. Box 94656 Washington, D.C. 20090-6456 Dear Mr. Pooler: On behalf of SQM North America, I am presenting this petition for the continued usage of non- synthetic Natural Sodium Nitrate in USDA Certified Organic crop production in The United States of America. Our product is necessary for our growers to maintain their economic viability; furthermore, this product is agronomically and environmentally sound and adheres to the principles of organic crop production. Natural Sodium Nitrate is permitted as a source of nitrogen for USDA Certified organic crops grown and used in The United States of America and this petition seeks to continue its usage. We look forward to the continued usage of Natural Sodium Nitrate and appreciate your attention to this petition. If you have any questions, please contact me. Sincerely, Bill McBride Director Sales US. and Canada SQM NORTH AMERICA CORP. 3101 Towercreek Parkway. Suite 450 Atlanta. GA 30330 Td: (1 - 770) 016 9400 Fa: (1 - 770) 016 9454 www.sam.cotq \A United States Agricultural STOP 0268 - Room 40084 Department of Marketing 1400 Independence Avenue, SW. i I Agriculture Service Washington, D.C. 20250-0200 February 9,2005 Bill McBride SQM North America, Corp. -
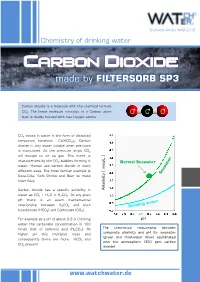
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

AP Chemistry Free Response
AP Chemistry Exam Reactions: Questions and Answers With the new format of the exam in 2007 and the availability of both questions and answers on the web at AP Central (http://apcentral.collegeboard.com:80/apc/public/courses/4606.html), I have determined not to update this page any longer. Please create an account as a teacher at AP Central and navigate to the full exams and scoring rubrics which are available back to 2003 Beginning in 2007, question 4 is no longer 5 out of 8 responses but rather three required responses. Also, in addition to writing the reactants and products, the equation must be balanced and there is a question about the chemical reaction. 2007 (a) A solution of sodium hydroxide is added to a solution of lead(II) nitrate. If 1.0 L volumes of 1.0 M solutions of sodium hydroxide and lead(II) nitrate are mixed together, now many moles of product(s) will be produced? Assume the reaction goes to completion. (b) Excess nitric acid is added to solid calcium carbonate. Briefly explain why statues made of marble (calcium carbonate) displayed outdoors in urban areas are deteriorating. (c) A solution containing silver(I) ion (an oxidixing agent) is mixed with a; solution containing iron(II) ion (a reducing agent). If the contents of the reaction mixture described above are filtered, what substance(s), if any, would remain on the filter paper? - 2+ → (a) (i) Balanced equation: 2OH + Pb Pb(OH)2 (s) (ii) The moles of each reactant are obtained by multiplying the volume times the molarity. -

A Review of the Patents and Literature on the Manufacture of Potassium Nitrate with Notes on Its Occurrence and Uses
UNITED STATES DEPARTMENT OF AGRICULTURE Miscellaneous Publication No. 192 Washington, D.C. July 1934 A REVIEW OF THE PATENTS AND LITERATURE ON THE MANUFACTURE OF POTASSIUM NITRATE WITH NOTES ON ITS OCCURRENCE AND USES By COLIN W. WHITTAKER. Associate Chemist and FRANK O. LUNDSTROM, Assistant Chemist Division of Fertilizer Technology, Fertilizer Investigationa Bureau of Chemistry and Soils For sale by the Superintendent of Documents, Washington, D.C. .....-..- Price 5 cents UNITED STATES DEPARTMENT OF AGRICULTURE Miscellaneous Publication No. 192 Washington, D.C. July 1934 A REVIEW OF THE PATENTS AND LITERATURE ON THE MANUFACTURE OF POTASSIUM NITRATE WITH NOTES ON ITS OCCURRENCE AND USES By COLIN W. WHITTAKER, associate chemist, and FRANK O. LUNDSTROM, assistant chemist, Division of Fertilizer Technology, Fertilizer Investigations, Bureau oj Chemistry and Soils CONTENTS Page Production of potassium nitrate —Contd. Page Processes involving dilute oxides of Introduction } nitrogen 22 Historical sketch 3 Absorption in carbonates, bicarbon- Statistics of the saltpeter industry 4 ates, or hydroxides 22 Potassium nitrate as a plant food 8 Conversion of nitrites to nitrates 23 Occurrence of potassium nitrate 9 Processes involving direct action of nitric Production of potassium nitrate 11 acid or oxides of nitrogen on potassium Saltpeter from the soil 11 compounds 23 In East India 11 Potassium bicarbonate and nitric In other countries 12 acid or ammonium nitrate 23 Chilean high-potash nitrate 13 Potassium hydroxide or carbonate Composting and