TRAIL in Cancer Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Katalog 2015 Cover Paul Lin *Hinweis Förderung.Indd
Product List 2015 WE LIVE SERVICE Certificates quartett owns two productions sites that are certified according to EN ISO 9001:2008 Quality management systems - Requirements EN ISO 13485:2012 + AC:2012 Medical devices - Quality management systems - Requirements for regulatory purposes GMP Conformity Our quality management guarantees products of highest quality! 2 Foreword to the quartett product list 2015 quartett Immunodiagnostika, Biotechnologie + Kosmetik Vertriebs GmbH welcomes you as one of our new business partners as well as all of our previous loyal clients. You are now member of quartett´s worldwide customers. First of all we would like to introduce ourselves to you. Founded as a family-run company in 1986, quartett ensures for more than a quarter of a century consistent quality of products. Service and support of our valued customers are our daily businesses. And we will continue! In the end 80´s quartett offered radioimmunoassay and enzyme immunoassay kits from different manufacturers in the USA. In the beginning 90´s the company changed its strategy from offering products for routine diagnostic to the increasing field of research and development. Setting up a production plant in 1997 and a second one in 2011 supported this decision. The company specialized its product profile in the field of manufacturing synthetic peptides for antibody production, peptides such as protease inhibitors, biochemical reagents and products for histology, cytology and immunohistology. All products are exclusively manufactured in Germany without outsourcing any production step. Nowadays, we expand into all other diagnostic and research fields and supply our customers in universities, government institutes, pharmaceutical and biotechnological companies, hospitals, and private doctor offices. -

TRAIL and Cardiovascular Disease—A Risk Factor Or Risk Marker: a Systematic Review
Journal of Clinical Medicine Review TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review Katarzyna Kakareko 1,* , Alicja Rydzewska-Rosołowska 1 , Edyta Zbroch 2 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (A.R.-R.); [email protected] (T.H.) 2 Department of Internal Medicine and Hypertension, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Abstract: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a pro-apoptotic protein showing broad biological functions. Data from animal studies indicate that TRAIL may possibly contribute to the pathophysiology of cardiomyopathy, atherosclerosis, ischemic stroke and abdomi- nal aortic aneurysm. It has been also suggested that TRAIL might be useful in cardiovascular risk stratification. This systematic review aimed to evaluate whether TRAIL is a risk factor or risk marker in cardiovascular diseases (CVDs) focusing on major adverse cardiovascular events. Two databases (PubMed and Cochrane Library) were searched until December 2020 without a year limit in accor- dance to the PRISMA guidelines. A total of 63 eligible original studies were identified and included in our systematic review. Studies suggest an important role of TRAIL in disorders such as heart failure, myocardial infarction, atrial fibrillation, ischemic stroke, peripheral artery disease, and pul- monary and gestational hypertension. Most evidence associates reduced TRAIL levels and increased TRAIL-R2 concentration with all-cause mortality in patients with CVDs. It is, however, unclear Citation: Kakareko, K.; whether low TRAIL levels should be considered as a risk factor rather than a risk marker of CVDs. -
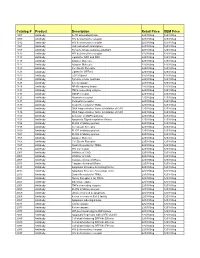
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

Dcr2 (Decoy Receptor 2, TRAIL-R4, TRUNDD)/Fc Chimera Human, Recombinant Expressed in Mouse NSO Cells
DcR2 (Decoy Receptor 2, TRAIL-R4, TRUNDD)/Fc Chimera Human, Recombinant Expressed in mouse NSO cells Product Number D9813 Product Description Reagents Recombinant human DcR2 (TRAIL-R4, TRUNDD) is a DcR2 is supplied as approximately 100 mg of protein chimeric protein expressed in mouse NSO cells. The lyophilized from a 0.2 mm filtered solution in phosphate 1, 2 extracellular domain of human DcR2 is fused to the buffered saline. carboxy-terminal 6X histidine-tagged Fc portion of human IgG1 by a polypeptide linker. Mature Preparation Instructions recombinant human DcR2 is a disulfide-linked Reconstitute the contents of the vial using sterile homodimeric protein. The reduced DcR2 monomer has phosphate-buffered saline (PBS) containing at least a molecular mass of approximately 44.2 kDa. Due to 0.1% human serum albumin or bovine serum albumin. glycosylation, recombinant human DcR2 migrates as an Prepare a stock solution of no less than 50 mg/ml. approximately 70-80 kDa protein in SDS-PAGE under reducing conditions. Storage/Stability Store at -20°C. Upon reconstitution, store at 2°-8°C for Apoptosis or programmed cell death is induced in cells one month. For extended storage, freeze in working by a group of death domain-containing receptors aliquots. Repeated freezing and thawing is not including TNFR1, Fas, DR3, DR4, and DR5. Binding of recommended. ligand to these receptors sends signals that activate members of the caspase family of proteases. The Product Profile signals ultimately cause the degradation of chromo- DcR2 is measured by its ability to neutralize apoptosis somal DNA by activating DNase. of mouse L929 cells treated with 50 ng/ml TRAIL. -

Biolegend.Com
Mechanisms of Cell Death TRAIL (TNFSF10) TNF-α Death Receptor 4 (TNFRSF10A/TRAIL-R1) Death Receptor 5 Zombie Dyes (TNFRSF10B/TRAIL-R2) Propidium Iodide (PI) BAT1, TIM-4 TNF RI (TNFRSF1A) 7-Amino-Actinomycin (7-AAD) MER TNF RII (TNFRSF1B) FAS-L GAS6 (TNFSF6/CD178) TRAIL (TNFSF10) Apoptotic Cell Death Domain Zombie Dyes Phosphatidylserine K63 Ubiquitin NH2 Removal ICAM3? ROCK1 NH CD14 2 Eat-Me Signals FAS Death Inducing Cytoskeletal Rearrangement, (TNFRSF6/CD95) Signaling Complex (DISC) TRADD Cytoskeletal Rearrangement, TRADD Decoy Receptor 2 FADD (TNFRSF10D/TRAIL-R4) Actomysin Contraction Engulfment RIP1 TWEAK RIP1 oxLDL (TNFSF12) FADD CIAP1/2 K63 Ubiquitination Blebbing CD36 Death Receptor 3 TWEAK (TNFSF12) PI FADD (TNFRSF25, APO-3) 7-AAD TRAF1 FADD Procaspase 8,10 TRAF 3 Phagocyte FLIP PANX1 Macrophage Monocyte Neutrophil Dendritic Cell Fibroblast Mast Cell Procaspase 8,10 NF-kB TWEAK-R (TNFRSF12A/Fn14) Find-Me Signals Lysophosphocholine C Caspase 8,10 TRAF5 TRAF2 Sphingosine-1-Phosphate G2A? Nucleotides A Decoy TRAIL Receptor R1 (TNFRSF23) Bid Cell Survival ATP, UTP Decoy TRAIL Receptor R2 (TNFRSF22) Sphingosine-1 TRADD Phosphate Receptor Decoy Receptor 1 (TNFRSF10C/TRAIL-R3) Procaspase 3 Proliferation RIP1 G P2y2 t-Bid Bcl-2 T Chemotaxis, Caspase 3 Bcl-2-xL, MCL-1 ? ICAD RIP1 Engulfment Degradation Bax, Bak Oligomerization TRADD Death Receptor 6 Extracellular ATP Bacterial pore-forming toxins TRAIL (TNFSF10) ICAD (TNFRSF21) Monosodium urate crystals Cholesterol crystals Death Receptor DNA Fragmentation Cholera toxin B, Mitochondria -

WO 2018/027204 Al 08 February 2018 (08.02.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/027204 Al 08 February 2018 (08.02.2018) W !P O PCT (51) International Patent Classification: only): F. HOFFMANN-LA ROCHE AG [CH/CH]; Gren- C07K 16/28 (2006.01) A61K 39/00 (2006.01) zacherstrasse 124, 4070 Basel (CH). (21) International Application Number: (72) Inventor; and PCT/US20 17/045642 (71) Applicant: HARRIS, Seth [US/US]; c/o Genentech, Inc., 1 DNA Way, South San Francisco, California 94080 (US). (22) International Filing Date: 04 August 2017 (04.08.2017) (72) Inventors: LAZAR, Greg; c/o Genentech, Inc., 1 DNA Way, South San Francisco, California 94080 (US). YANG, (25) Filing Language: English Yanli; c/o Genentech, Inc., 1 DNA Way, South San Fran (26) Publication Language: English cisco, California 94080 (US). CHRISTENSEN, Erin H.; c/ o Genentech, Inc., 1 DNA Way, South San Francisco, Cali (30) Priority Data: fornia 94080 (US). HANG, Julie; 6606 Wisteria Way, San 62/371,671 05 August 2016 (05.08.2016) US Jose, California 95 129 (US). KIM, Jeong; c/o Genentech, (71) Applicant (for all designated States except AL, AT, BA, BE, Inc., 1 DNA Way, South San Francisco, California 94080 BG, CH, CN, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, (US). HR, HU, IE, IN, IS, IT, LT, LU, LV, MC, MK, MT, NL, (74) Agent: JONES, Kevin et al; Morrison & Foerster LLP, NO, PL, PT RO, RS, SE, SI, SK, SM, TR): GENENTECH, 425 Market Street, San Francisco, California 94105-2482 INC. -

Supplementary Tables S1-S3
Supplementary Table S1: Real time RT-PCR primers COX-2 Forward 5’- CCACTTCAAGGGAGTCTGGA -3’ Reverse 5’- AAGGGCCCTGGTGTAGTAGG -3’ Wnt5a Forward 5’- TGAATAACCCTGTTCAGATGTCA -3’ Reverse 5’- TGTACTGCATGTGGTCCTGA -3’ Spp1 Forward 5'- GACCCATCTCAGAAGCAGAA -3' Reverse 5'- TTCGTCAGATTCATCCGAGT -3' CUGBP2 Forward 5’- ATGCAACAGCTCAACACTGC -3’ Reverse 5’- CAGCGTTGCCAGATTCTGTA -3’ Supplementary Table S2: Genes synergistically regulated by oncogenic Ras and TGF-β AU-rich probe_id Gene Name Gene Symbol element Fold change RasV12 + TGF-β RasV12 TGF-β 1368519_at serine (or cysteine) peptidase inhibitor, clade E, member 1 Serpine1 ARE 42.22 5.53 75.28 1373000_at sushi-repeat-containing protein, X-linked 2 (predicted) Srpx2 19.24 25.59 73.63 1383486_at Transcribed locus --- ARE 5.93 27.94 52.85 1367581_a_at secreted phosphoprotein 1 Spp1 2.46 19.28 49.76 1368359_a_at VGF nerve growth factor inducible Vgf 3.11 4.61 48.10 1392618_at Transcribed locus --- ARE 3.48 24.30 45.76 1398302_at prolactin-like protein F Prlpf ARE 1.39 3.29 45.23 1392264_s_at serine (or cysteine) peptidase inhibitor, clade E, member 1 Serpine1 ARE 24.92 3.67 40.09 1391022_at laminin, beta 3 Lamb3 2.13 3.31 38.15 1384605_at Transcribed locus --- 2.94 14.57 37.91 1367973_at chemokine (C-C motif) ligand 2 Ccl2 ARE 5.47 17.28 37.90 1369249_at progressive ankylosis homolog (mouse) Ank ARE 3.12 8.33 33.58 1398479_at ryanodine receptor 3 Ryr3 ARE 1.42 9.28 29.65 1371194_at tumor necrosis factor alpha induced protein 6 Tnfaip6 ARE 2.95 7.90 29.24 1386344_at Progressive ankylosis homolog (mouse) -

Decoy TRAIL Receptor CD264: a Cell Surface Marker of Cellular Aging for Human Bone Marrow-Derived Mesenchymal Stem Cells Sean D
Madsen et al. Stem Cell Research & Therapy (2017) 8:201 DOI 10.1186/s13287-017-0649-4 RESEARCH Open Access Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells Sean D. Madsen1,2,3, Katie C. Russell1,2,3, H. Alan Tucker3, Julie Glowacki4, Bruce A. Bunnell2,3,5 and Kim C. O’Connor1,2,3,5* Abstract Background: Mesenchymal stem cells (MSCs) are a mixture of progenitors that are heterogeneous in their regenerative potential. Development of MSC therapies with consistent efficacy is hindered by the absence of an immunophenotype of MSC heterogeneity. This study evaluates decoy TRAIL receptor CD264 as potentially the first surface marker to detect cellular aging in heterogeneous MSC cultures. Methods: CD264 surface expression, regenerative potential, and metrics of cellular aging were assessed in vitro formarrowMSCsfrom12donorsages20–60 years old. Male and female donors were age matched. Expression of CD264 was compared with that of p16, p21, and p53 during serial passage of MSCs. Results: When CD264+ cell content was 20% to 35%, MSC cultures from young (ages 20–40 years) and older (ages 45–60 years) donors proliferated rapidly and differentiated extensively. Older donor MSCs containing < 35% CD264+ cells had a small size and negligible senescence despite the donor’s advanced chronological age. Above the 35% threshold, CD264 expression inversely correlated with proliferation and differentiation potential. When CD264+ cell content was 75%, MSCs were enlarged and mostly senescent with severely compromised regenerative potential. There was no correlation of the older donors’ chronological age to either CD264+ cell content or the regenerative potential of the donor MSCs. -

Anti-Dcr2 (D3188)
Anti-DcR2 produced in rabbit, affinity isolated antibody Catalog Number D3188 Synonyms: Anti-Decoy Receptor 2; Anti-Trail -R4; Storage/Stability Anti-TRUNDD Antibody can be stored at 2-8 °C for three months and at −20 °C for one year. As with all antibodies, care Product Description should be taken to avoid repeated freeze thaw cycles. Anti-DcR2 is produced in rabbit using as immunogen a Antibodies should not be exposed to prolonged high peptide corresponding to amino acids 249-263 of temperatures. human DcR2 precursor. Product Profile Anti-DcR2 recognizes DcR2 by immunoblotting. Immunoblotting: DcR2 antibody can be used for detection of DcR2 expression by Western blot at Apoptosis or programmed cell death is induced in cells 1 μg/mL. A band of ~36 kDa is detected. by a group of death domain-containing receptors including TNFR1, Fas, DR3, DR4, and DR5. Binding of Note: In order to obtain best results in different ligand to these receptors sends signals that activate techniques and preparations we recommend members of the caspase family of proteases. The determining optimal working concentration by titration signals ultimately cause the degradation of test. chromosomal DNA by activating DNase. DR5 and DR4 are closely related members of the TNF family of death- References domain containing receptors. These proteins share 1. Singh, et al., Death domain receptors and their role homologies in both their extracellular ligand binding in cell demise. J. Interferon Cytokine Res., 18, 439- domains and their intracellular effector domains (death 450 (1998). domains). These death domains are ~80 amino acids 2. Pan G, et al., The receptor for the cytotoxic ligand long.1 Both DR4 and DR5 bind TRAIL. -

Cell Structure & Function
Cell Structure & Function Antibodies and Reagents BioLegend is ISO 13485:2016 Certified Toll-Free Tel: (US & Canada): 1.877.BIOLEGEND (246.5343) Tel: 858.768.5800 biolegend.com 02-0012-03 World-Class Quality | Superior Customer Support | Outstanding Value Table of Contents Introduction ....................................................................................................................................................................................3 Cell Biology Antibody Validation .............................................................................................................................................4 Cell Structure/ Organelles ..........................................................................................................................................................8 Cell Development and Differentiation ................................................................................................................................10 Growth Factors and Receptors ...............................................................................................................................................12 Cell Proliferation, Growth, and Viability...............................................................................................................................14 Cell Cycle ........................................................................................................................................................................................16 Cell Signaling ................................................................................................................................................................................18 -

Sensitization for Death Receptor- Or Drug-Induced Apoptosis by Re-Expression of Caspase-8 Through Demethylation Or Gene Transfer
Oncogene (2001) 20, 5865 ± 5877 ã 2001 Nature Publishing Group All rights reserved 0950 ± 9232/01 $15.00 www.nature.com/onc Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer Simone Fulda1, Martin U KuÈ fer1, Eric Meyer1, Frans van Valen2, Barbara Dockhorn-Dworniczak3 and Klaus-Michael Debatin*,1 1University Children's Hospital, Prittwitzstr. 43, D-89075 Ulm, Germany; 2Laboratory for Experimental Orthopedic Research, WestfaÈlische-Wilhelms University, D-48149 MuÈnster, Germany; 3Department of Pathology, WestfaÈlische-Wilhelms University, D-48149 MuÈnster, Germany Resistance of tumors to treatment with cytotoxic drugs, Introduction irradiation or immunotherapy may be due to disrupted apoptosis programs. Here, we report in a variety of Cell death by apoptosis plays a pivotal role in the dierent tumor cells including Ewing tumor, neuroblas- regulation of various physiological or pathological toma, malignant brain tumors and melanoma that conditions and has also been implied to mediate caspase-8 expression acts as a key determinant of therapy-induced cytotoxicity, e.g. in response to sensitivity for apoptosis induced by death-inducing cytotoxic drug treatment or g-irradiation (Peter and ligands or cytotoxic drugs. In tumor cell lines resistant Krammer, 1998; Grith and Lynch, 1998; Kaufmann to TRAIL, anti-CD95 or TNFa, caspase-8 protein and and Earnshaw, 2000; Debatin, 1997; Friesen et al., mRNA expression was decreased or absent without 1996; Fulda et al., 1997, 1998a,b). Apoptosis pathways caspase-8 gene loss. Methylation-speci®c PCR revealed may be initiated through dierent entry sites, such as hypermethylation of caspase-8 regulatory sequences in death receptors (receptor pathway) or mitochondria cells with impaired caspase-8 expression. -

Cell-Mediated and Cell Membrane-Coated Nanoparticles for Drug Delivery and Cancer Therapy
Yaman et al. Cancer Drug Resist 2020;3:879-911 Cancer DOI: 10.20517/cdr.2020.55 Drug Resistance Review Open Access Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy Serkan Yaman1,2,#, Uday Chintapula1,2,#, Edgar Rodriguez1, Harish Ramachandramoorthy1,2, Kytai T. Nguyen1,2 1Department of Bioengineering, University of Texas at Arlington, Arlington, TX 76010, USA. 2Joint Bioengineering Program, University of Texas Southwestern Medical Center, Dallas, TX 75235, USA. #Yaman S and Chintapula U contributed equally to this work. Correspondence to: Dr. Kytai T. Nguyen, Department of Bioengineering, University of Texas at Arlington, 500 UTA Blvd ERB244, Arlington, TX 76010, USA. E-mail: [email protected] How to cite this article: Yaman S, Chintapula U, Rodriguez E, Ramachandramoorthy H, Nguyen KT. Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy. Cancer Drug Resist 2020;3:879-911. http://dx.doi.org/10.20517/cdr.2020.55 Received: 24 Jul 2020 First Decision: 25 Aug 2020 Revised: 16 Sep 2020 Accepted: 21 Sep 2020 Available online: 3 Nov 2020 Academic Editor: Vladimir P. Torchilin Copy Editor: Cai-Hong Wang Production Editor: Jing Yu Abstract Nanotechnology-based drug delivery platforms have been developed over the last two decades because of their favorable features in terms of improved drug bioavailability and stability. Despite recent advancement in nanotechnology platforms, this approach still falls short to meet the complexity of biological systems and diseases, such as avoiding systemic side effects, manipulating biological interactions and overcoming drug resistance, which hinders the therapeutic outcomes of the NP-based drug delivery systems.