University of Groningen Probing the Ligand Receptor
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

RANKL As a Novel EMT Marker 858 Cell Research (2008) 18:858-870
npg RANKL as a novel EMT marker 858 Cell Research (2008) 18:858-870. npg © 2008 IBCB, SIBS, CAS All rights reserved 1001-0602/08 $ 30.00 ORIGINAL ARTICLE www.nature.com/cr Receptor activator of NF-κB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells Valerie A Odero-Marah1, Ruoxiang Wang1, Gina Chu1, Majd Zayzafoon2, Jianchun Xu1, Chunmeng Shi1, Fray F Marshall1, Haiyen E Zhau1, Leland WK Chung1 1Molecular Urology and Therapeutics Program, Department of Urology and Winship Cancer Institute, Emory University School of Medicine, 1365B Clifton Road, NE, Atlanta, GA 30322, USA; 2Department of Pathology, University of Alabamn, Birmingham, AL 35294, USA Epithelial-mesenchymal transition (EMT) in cancer describes the phenotypic and behavioral changes of cancer cells from indolent to virulent forms with increased migratory, invasive and metastatic potential. EMT can be induced by soluble proteins like transforming growth factor β1 (TGFβ1) and transcription factors including Snail and Slug. We uti- lized the ARCaPE/ARCaPM prostate cancer progression model and LNCaP clones stably overexpressing Snail to identify novel markers associated with EMT. Compared to ARCaPE cells, the highly tumorigenic mesenchymal ARCaPM and ARCaPM1 variant cells displayed a higher incidence of bone metastasis after intracardiac administration in SCID mice. ARCaPM and ARCaPM1 expressed mesenchymal stromal markers of vimentin and N-cadherin in addition to elevated levels of Receptor Activator of NF-κB Ligand (RANKL). We observed that both epidermal growth factor (EGF) plus TGFβ1 treatment and Snail overexpression induced EMT in ARCaPE and LNCaP cells, and EMT was associated with increased expression of RANKL protein. -

Katalog 2015 Cover Paul Lin *Hinweis Förderung.Indd
Product List 2015 WE LIVE SERVICE Certificates quartett owns two productions sites that are certified according to EN ISO 9001:2008 Quality management systems - Requirements EN ISO 13485:2012 + AC:2012 Medical devices - Quality management systems - Requirements for regulatory purposes GMP Conformity Our quality management guarantees products of highest quality! 2 Foreword to the quartett product list 2015 quartett Immunodiagnostika, Biotechnologie + Kosmetik Vertriebs GmbH welcomes you as one of our new business partners as well as all of our previous loyal clients. You are now member of quartett´s worldwide customers. First of all we would like to introduce ourselves to you. Founded as a family-run company in 1986, quartett ensures for more than a quarter of a century consistent quality of products. Service and support of our valued customers are our daily businesses. And we will continue! In the end 80´s quartett offered radioimmunoassay and enzyme immunoassay kits from different manufacturers in the USA. In the beginning 90´s the company changed its strategy from offering products for routine diagnostic to the increasing field of research and development. Setting up a production plant in 1997 and a second one in 2011 supported this decision. The company specialized its product profile in the field of manufacturing synthetic peptides for antibody production, peptides such as protease inhibitors, biochemical reagents and products for histology, cytology and immunohistology. All products are exclusively manufactured in Germany without outsourcing any production step. Nowadays, we expand into all other diagnostic and research fields and supply our customers in universities, government institutes, pharmaceutical and biotechnological companies, hospitals, and private doctor offices. -

TNF Decoy Receptors Encoded by Poxviruses
pathogens Review TNF Decoy Receptors Encoded by Poxviruses Francisco Javier Alvarez-de Miranda † , Isabel Alonso-Sánchez † , Antonio Alcamí and Bruno Hernaez * Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas, Campus de Cantoblanco, Universidad Autónoma de Madrid, Nicolás Cabrera 1, 28049 Madrid, Spain; [email protected] (F.J.A.-d.M.); [email protected] (I.A.-S.); [email protected] (A.A.) * Correspondence: [email protected]; Tel.: +34-911-196-4590 † These authors contributed equally. Abstract: Tumour necrosis factor (TNF) is an inflammatory cytokine produced in response to viral infections that promotes the recruitment and activation of leukocytes to sites of infection. This TNF- based host response is essential to limit virus spreading, thus poxviruses have evolutionarily adopted diverse molecular mechanisms to counteract TNF antiviral action. These include the expression of poxvirus-encoded soluble receptors or proteins able to bind and neutralize TNF and other members of the TNF ligand superfamily, acting as decoy receptors. This article reviews in detail the various TNF decoy receptors identified to date in the genomes from different poxvirus species, with a special focus on their impact on poxvirus pathogenesis and their potential use as therapeutic molecules. Keywords: poxvirus; immune evasion; tumour necrosis factor; tumour necrosis factor receptors; lymphotoxin; inflammation; cytokines; secreted decoy receptors; vaccinia virus; ectromelia virus; cowpox virus Citation: Alvarez-de Miranda, F.J.; Alonso-Sánchez, I.; Alcamí, A.; 1. TNF Biology Hernaez, B. TNF Decoy Receptors TNF is a potent pro-inflammatory cytokine with a broad range of biological effects, Encoded by Poxviruses. Pathogens ranging from the activation of inflammatory gene programs to cell differentiation or 2021, 10, 1065. -

RANK Interaction and Signaling − RANKL Structural and Functional
Structural and Functional Insights of RANKL −RANK Interaction and Signaling Changzhen Liu, Thomas S. Walter, Peng Huang, Shiqian Zhang, Xuekai Zhu, Ying Wu, Lucy R. Wedderburn, Peifu This information is current as Tang, Raymond J. Owens, David I. Stuart, Jingshan Ren and of October 1, 2021. Bin Gao J Immunol published online 14 May 2010 http://www.jimmunol.org/content/early/2010/05/14/jimmun ol.0904033 Downloaded from Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: by guest on October 1, 2021 http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published May 14, 2010, doi:10.4049/jimmunol.0904033 The Journal of Immunology Structural and Functional Insights of RANKL–RANK Interaction and Signaling Changzhen Liu,*,†,1 Thomas S. Walter,‡,1 Peng Huang,x Shiqian Zhang,{ Xuekai Zhu,*,† Ying Wu,*,† Lucy R. Wedderburn,‖ Peifu Tang,x Raymond J. Owens,‡ David I. Stuart,‡ Jingshan Ren,‡ and Bin Gao*,†,‖ Bone remodeling involves bone resorption by osteoclasts and synthesis by osteoblasts and is tightly regulated by the receptor activator of the NF-kB ligand (RANKL)/receptor activator of the NF-kB (RANK)/osteoprotegerin molecular triad. -

TRAIL and Cardiovascular Disease—A Risk Factor Or Risk Marker: a Systematic Review
Journal of Clinical Medicine Review TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review Katarzyna Kakareko 1,* , Alicja Rydzewska-Rosołowska 1 , Edyta Zbroch 2 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (A.R.-R.); [email protected] (T.H.) 2 Department of Internal Medicine and Hypertension, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Abstract: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a pro-apoptotic protein showing broad biological functions. Data from animal studies indicate that TRAIL may possibly contribute to the pathophysiology of cardiomyopathy, atherosclerosis, ischemic stroke and abdomi- nal aortic aneurysm. It has been also suggested that TRAIL might be useful in cardiovascular risk stratification. This systematic review aimed to evaluate whether TRAIL is a risk factor or risk marker in cardiovascular diseases (CVDs) focusing on major adverse cardiovascular events. Two databases (PubMed and Cochrane Library) were searched until December 2020 without a year limit in accor- dance to the PRISMA guidelines. A total of 63 eligible original studies were identified and included in our systematic review. Studies suggest an important role of TRAIL in disorders such as heart failure, myocardial infarction, atrial fibrillation, ischemic stroke, peripheral artery disease, and pul- monary and gestational hypertension. Most evidence associates reduced TRAIL levels and increased TRAIL-R2 concentration with all-cause mortality in patients with CVDs. It is, however, unclear Citation: Kakareko, K.; whether low TRAIL levels should be considered as a risk factor rather than a risk marker of CVDs. -

The Roadmap of RANKL/RANK Pathway in Cancer
cells Review The Roadmap of RANKL/RANK Pathway in Cancer Sandra Casimiro 1,* , Guilherme Vilhais 2, Inês Gomes 1 and Luis Costa 1,3,* 1 Luis Costa Laboratory, Instituto de Medicina Molecular—João Lobo Antunes, Faculdade de Medicina da Universidade de Lisboa, 1649-028 Lisboa, Portugal; [email protected] 2 Faculdade de Medicina da Universidade de Lisboa, 1649-028 Lisboa, Portugal; [email protected] 3 Oncology Division, Hospital de Santa Maria, Centro Hospitalar Universitário Lisboa Norte, 1649-028 Lisboa, Portugal * Correspondence: [email protected] (S.C.); [email protected] (L.C.) Abstract: The receptor activator of the nuclear factor-κB ligand (RANKL)/RANK signaling pathway was identified in the late 1990s and is the key mediator of bone remodeling. Targeting RANKL with the antibody denosumab is part of the standard of care for bone loss diseases, including bone metastases (BM). Over the last decade, evidence has implicated RANKL/RANK pathway in hormone and HER2-driven breast carcinogenesis and in the acquisition of molecular and phenotypic traits associated with breast cancer (BCa) aggressiveness and poor prognosis. This marked a new era in the research of the therapeutic use of RANKL inhibition in BCa. RANKL/RANK pathway is also an important immune mediator, with anti-RANKL therapy recently linked to improved response to immunotherapy in melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC). This review summarizes and discusses the pre-clinical and clinical evidence of the relevance of the RANKL/RANK pathway in cancer biology and therapeutics, focusing on bone metastatic disease, BCa onset and progression, and immune modulation. -

The Role of the Immune Checkpoint Modulator OX40 and Its Ligand in NK Cell Immunosurveillance and Acute Myeloid Leukemia
1 The role of the immune checkpoint modulator OX40 and its ligand in NK cell 2 immunosurveillance and acute myeloid leukemia 3 Running title: OX40-OX40L in NK cell immunosurveillance 4 Keywords: OX40, AML, NK cells, immunotherapy, checkpoint 5 Tina Nuebling*1, Carla Emilia Schumacher*1,2, Martin Hofmann3, Ilona Hagelstein1, Benjamin Joachim 6 Schmiedel1, Stefanie Maurer1, Birgit, Federmann4, Kathrin Rothfelder1, Malte Roerden2, Daniela Dörfel1,2, 7 Pascal Schneider5, Gundram Jung3, Helmut Rainer Salih1,2 8 1 9 Clinical Collaboration Unit Translational Immunology, German Cancer Consortium (DKTK) and German 10 Cancer Research Center (DKFZ), Heidelberg, Germany 2 11 Department of Hematology and Oncology, Eberhard Karls University, Tuebingen, Germany 3 12 Department of Immunology, Eberhard Karls University, Tuebingen, Germany 13 4 Department of Pathology, Eberhard Karls University, Tuebingen, Germany 14 5 Department of Biochemistry, Epalinges, Switzerland 15 16 *these authors contributed equally to this work 17 18 Corresponding Author: 19 Helmut R. Salih, M.D. 20 Clinical Collaboration Unit Translational Immunology, German Cancer Consortium (DKTK) and German 21 Cancer Research Center (DKFZ) 22 Department of Hematology and Oncology, Eberhard Karls University 23 Otfried-Mueller Str. 10, 72076 Tuebingen, Germany 24 Phone: +49-7071-2983275; Fax: +49-7071-293671; Email: [email protected] 25 26 Funding: Supported by grants from the Deutsche Forschungsgemeinschaft (NU341/1-1, SA1360/7-3, 27 SFB685, project A07), Deutsche Krebshilfe (projects 111828 and 111134). PS is supported by grants from 28 the Swiss National Science Foundation. 29 Conflict-of-interest disclosure: The authors declare no potential conflicts of interest. 30 31 32 Text word count: 4347; Abstract word count: 238 33 5 figures, 2 tables, 4 supplementary figures 34 References: 50 1 35 Abstract 36 The TNF receptor family member OX40 promotes activation and proliferation of T cells, which 37 fuels present attempts to modulate this immune checkpoint to reinforce anti-tumor immunity. -

(Blys) on Diabetes-Related Periodontitis
ORIGINAL RESEARCH Immunology Preliminary findings on the possible role of B-lymphocyte stimulator (BLyS) on diabetes-related periodontitis Marx Haddley Ferreira Abstract: The possible role of B-cell growth and differentiation- DRUMOND(a) related cytokines on the pathogenesis of diabetes-related periodontitis Luciano Eduardo PUHL(a) has not been addressed so far. The aim of this study was to evaluate Poliana Mendes DUARTE(b) the effects of diabetes mellitus (DM) on the gene expression of Tamires Szeremeske de proliferation-inducing ligand (APRIL) and B-lymphocyte stimulator MIRANDA(c) (BLyS), two major cytokines associated to survival, differentiation Juliana Trindade and maturation of B cells in biopsies from gingival tissue with CLEMENTE-NAPIMOGA(a) periodontitis. Gingival biopsies were obtained from subjects with Daiane Cristina PERUZZO(a) periodontitis (n = 17), with periodontitis and DM (n = 19) as well as Elizabeth Ferreira MARTINEZ(a) from periodontally and systemically healthy controls (n = 10). Gene Marcelo Henrique NAPIMOGA(a) expressions for APRIL, BLyS, RANKL, OPG, TRAP and DC-STAMP were evaluated using qPCR. The expressions APRIL, BLyS, RANKL, (a) Faculdade São Leopoldo Mandic, Instituto OPG, TRAP and DC-STAMP were all higher in both periodontitis de Pesquisas São Leopoldo Mandic, groups when compared to the control group (p < 0.05). Furthermore, Campinas, SP, Brazil. the expressions of BLyS, TRAP and RANKL were significantly higher (b) University of Florida, College of Dentistry, in the subjects with periodontitis and DM when compared to those Department of Periodontology, Gainesville, FL, USA. with periodontitis alone (p < 0.05). The mRNA levels of BLyS correlated positively with RANKL in the subjects with periodontitis and DM (c) Guarulhos University, Dental Research Division, Department of Periodontology, São (p < 0.05). -

Multimeric Forms of 4-1BBL As Stimulators of T Cells for Adoptive Immunotherapy
Kornbluth et al. Journal for ImmunoTherapy of Cancer 2014, 2(Suppl 3):P246 http://www.immunotherapyofcancer.org/content/2/S3/P246 POSTERPRESENTATION Open Access Multimeric forms of 4-1BBL as stimulators of T cells for adoptive immunotherapy Richard S Kornbluth1*, Victoria Snarsky1, Geoffrey W Stone2 From Society for Immunotherapy of Cancer 29th Annual Meeting National Harbor, MD, USA. 6-9 November 2014 Members of the TNF SuperFamily of ligands (TNFSFs) Authors’ details 1Multimeric Biotherapeutics, Inc., La Jolla, CA, USA. 2University of Miami, have significant potential as immuno-oncology therapeu- Miller School of Medicine, Miami, FL, USA. tic agents. The TNFSFs are trimeric membrane proteins that can be cleaved into soluble single trimers. While Published: 6 November 2014 the soluble single trimers can be easily prepared and studied, they have little or no activity in vivo. This defi- ciency is caused by the need to cluster their cognate doi:10.1186/2051-1426-2-S3-P246 Cite this article as: Kornbluth et al.: Multimeric forms of 4-1BBL as receptors in the plane of the membrane in order to stimulators of T cells for adoptive immunotherapy. Journal for induce a supramolecular signaling complex on the cyto- ImmunoTherapy of Cancer 2014 2(Suppl 3):P246. plasmic side of the plasma membrane. For the TNFSF ligands, this requires that they be used as many-trimer multimers that mimic the natural expression of many trimers on the surface of stimulating cells. To meet this need, we prepared fusion proteins comprised of the extracellular domains of TNFSF ligands joined to a nat- ural protein that provides a multimerization scaffold. -

Belongs to the TNF Family a New Class of Reverse Signaling
A New Class of Reverse Signaling Costimulators Belongs to the TNF Family Mingyi Sun and Pamela J. Fink This information is current as J Immunol 2007; 179:4307-4312; ; of September 27, 2021. doi: 10.4049/jimmunol.179.7.4307 http://www.jimmunol.org/content/179/7/4307 References This article cites 85 articles, 38 of which you can access for free at: Downloaded from http://www.jimmunol.org/content/179/7/4307.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision http://www.jimmunol.org/ • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: by guest on September 27, 2021 http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2007 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. THE JOURNAL OF IMMUNOLOGY BRIEF REVIEWS A New Class of Reverse Signaling Costimulators Belongs to the TNF Family Mingyi Sun and Pamela J. Fink1 Recent evidence shows that many molecules of the TNF still remains unclear (7). The N-terminal cytoplasmic domains family serve as counter-receptors, inducing costimulation of most TNF family members are conserved across species but through reverse signals in addition to delivering signals not between family members, suggesting that the intracellular through their respective TNF receptors. -
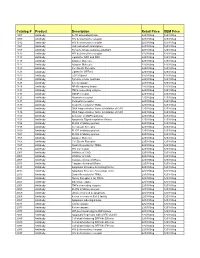
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

Dcr2 (Decoy Receptor 2, TRAIL-R4, TRUNDD)/Fc Chimera Human, Recombinant Expressed in Mouse NSO Cells
DcR2 (Decoy Receptor 2, TRAIL-R4, TRUNDD)/Fc Chimera Human, Recombinant Expressed in mouse NSO cells Product Number D9813 Product Description Reagents Recombinant human DcR2 (TRAIL-R4, TRUNDD) is a DcR2 is supplied as approximately 100 mg of protein chimeric protein expressed in mouse NSO cells. The lyophilized from a 0.2 mm filtered solution in phosphate 1, 2 extracellular domain of human DcR2 is fused to the buffered saline. carboxy-terminal 6X histidine-tagged Fc portion of human IgG1 by a polypeptide linker. Mature Preparation Instructions recombinant human DcR2 is a disulfide-linked Reconstitute the contents of the vial using sterile homodimeric protein. The reduced DcR2 monomer has phosphate-buffered saline (PBS) containing at least a molecular mass of approximately 44.2 kDa. Due to 0.1% human serum albumin or bovine serum albumin. glycosylation, recombinant human DcR2 migrates as an Prepare a stock solution of no less than 50 mg/ml. approximately 70-80 kDa protein in SDS-PAGE under reducing conditions. Storage/Stability Store at -20°C. Upon reconstitution, store at 2°-8°C for Apoptosis or programmed cell death is induced in cells one month. For extended storage, freeze in working by a group of death domain-containing receptors aliquots. Repeated freezing and thawing is not including TNFR1, Fas, DR3, DR4, and DR5. Binding of recommended. ligand to these receptors sends signals that activate members of the caspase family of proteases. The Product Profile signals ultimately cause the degradation of chromo- DcR2 is measured by its ability to neutralize apoptosis somal DNA by activating DNase. of mouse L929 cells treated with 50 ng/ml TRAIL.