AB 2588 EICG Appendix a Combined List of Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent ‘ Patented Mar
r 2,786,869 United States Patent ‘ Patented Mar. 26, 1957 l 2 tically, mixtures of tert-alkylamine such as are available on the market. Typical mixtures are those containing ‘ 2,786,869 C12H25NH2 to C15H31NH2 or C18H3'INH2 to C24H49NH2 N-TRIALKYLCARBINYL-N-(HYDROXYETHYL or C15H31- to C24H49NH2. These may be represented by POLYOXYETHYL) GLYCINES the formula Peter L. de Benneville and Homer J. Sims, Philadelphia, 31 Pa., assignors to Rohrn & Haas Company, Philadelphia, R’-—-C—NH2 Pa., a corporation of Delaware Rs No Drawing. Application June 16, 1954, 10 As catalysts in the ?rst step of the process of this Serial No. 437,273 invention, wherein the hydroxyethyl group is introduced, 9 Claims.‘ (or. 260-534) there may be used any of the strong acids, such as hy drochloric, hydrobromic, sulfuric, arylsulfonic, alkanesul fonic, or phosphoric. The preferred amount of this cat This invention relatesto-compounds of the structure 15 alyst is 10 to 30 mole percent of the amine. With R1 '(CH2CH2O),.H ‘ , amines from 12 carbon atoms upward it is exceedingly di?icult to introduce more than one hydroxyethyl group in a tert-alkylamine molecule. Such amines‘yicld ?nal 3 \CHrCOOH products which have the desired balance of properties. ' wherein R1, R2, and R3 are alkyl groups containing a 20 The ?rst reaction with ethylene oxide is effected by total of 11 to 23 carbon atoms and n is an integer having bringing together ethylene oxide and tert-alkylamine, a value from 5 to about 50 or more, preferably 5 to 25. usually by passing ethylene oxide into amine and catalyst, These compounds may be called N-(trialkylcarbinyl)-N at temperatures from 0° to 180° C. -

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Guaiana, G., Barbui, C., Caldwell, DM, Davies, SJC, Furukawa, TA
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Explore Bristol Research Guaiana, G., Barbui, C., Caldwell, D. M., Davies, S. J. C., Furukawa, T. A., Imai, H., ... Cipriani, A. (2017). Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis. Cochrane Database of Systematic Reviews, 2017(7), [CD012729]. https://doi.org/10.1002/14651858.CD012729 Publisher's PDF, also known as Version of record Link to published version (if available): 10.1002/14651858.CD012729 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Cochrane Library at https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012729/full . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/pure/about/ebr-terms Cochrane Database of Systematic Reviews Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis (Protocol) Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A. Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 7. -
Lab Standard Operating Procedures (Sops)
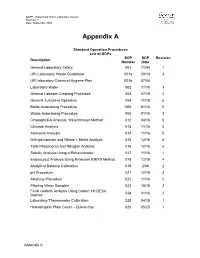
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian's, Malta
Clinical Toxicology ISSN: 1556-3650 (Print) 1556-9519 (Online) Journal homepage: http://www.tandfonline.com/loi/ictx20 XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian's, Malta To cite this article: (2015) XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian's, Malta, Clinical Toxicology, 53:4, 233-403, DOI: 10.3109/15563650.2015.1024953 To link to this article: http://dx.doi.org/10.3109/15563650.2015.1024953 Published online: 26 Mar 2015. Submit your article to this journal Article views: 3422 View related articles View Crossmark data Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ictx20 Download by: [UPSTATE Medical University Health Sciences Library] Date: 28 December 2016, At: 10:31 Clinical Toxicology (2015), 53, 233–403 Copyright © 2015 Informa Healthcare USA, Inc. ISSN: 1556-3650 print / 1556-9519 online DOI: 10.3109/15563650.2015.1024953 ABSTRACTS XXXV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 26–29 May 2015, St Julian ’ s, Malta 1. Modelling dose-concentration-response Introduction: The American Association of Poison Control Cen- ters (AAPCC) published its fi rst annual report in 1983. Call data Ursula Gundert-Remy from sixteen US poison centers was chronicled in that report. Seven submitted data for the entire year. By July 2000, 63 centers Institute for Clinical Pharmacology and Toxicology, Charit é were part of the national poison center system, but only 59 submit- Medical School, Berlin, Germany ted data for the full year. -

Theoretical Problem Icho 2018
19th – 29th July 2018 Bratislava, SLOVAKIA Prague, CZECH REPUBLIC www.50icho.eu THEORETICAL PROBLEMS Country: Name as in passport: Student code: Language: 50th IChO 2018 International Chemistry Olympiad SLOVAKIA & CZECH REPUBLIC BACK TO WHERE IT ALL BEGAN XXX-X INTERNATIONAL CHEMISTRY OLYMPIAD / SLOVAKIA & CZECH REPUBLIC, 2018 Table of Contents Instructions ...................................................................................................................................... 2 Physical constants and equations ................................................................................................... 3 Problem 1. DNA .............................................................................................................................. 5 Problem 2. Repatriation of remains in the middle ages .................................................................. 14 Problem 3. Emerging electro-mobility ............................................................................................ 22 Problem 4. Column chromatography of radioactive copper ........................................................... 30 Problem 5. Bohemian garnet ......................................................................................................... 34 Problem 6. Let’s go mushrooming ................................................................................................. 41 Problem 7. Cidofovir ...................................................................................................................... 47 -

Re-Visiting Hypersensitivity Reactions to Taxanes: a Comprehensive Review
Clinic Rev Allerg Immunol DOI 10.1007/s12016-014-8416-0 Re-visiting Hypersensitivity Reactions to Taxanes: A Comprehensive Review Matthieu Picard & Mariana C. Castells # Springer Science+Business Media New York 2014 Abstract Taxanes (a class of chemotherapeutic agents) Keywords Taxane . Paclitaxel . Taxol . Docetaxel . are an important cause of hypersensitivity reactions Taxotere . Nab-paclitaxel . Abraxane . Cabazitaxel . (HSRs) in cancer patients. During the last decade, the Chemotherapy . Hypersensitivity . Allergy . Skin test . development of rapid drug desensitization has been key Desensitization . Challenge . Diagnosis . Review . IgE . to allow patients with HSRs to taxanes to be safely re- Complement . Mechanism treated although the mechanisms of these HSRs are not fully understood. Earlier studies suggested that solvents, such as Cremophor EL used to solubilize paclitaxel, Introduction were responsible for HSRs through complement activa- tion, but recent findings have raised the possibility that Hypersensitivity reactions (HSRs) to chemotherapy are in- some of these HSRs are IgE-mediated. Taxane skin creasingly common and represent an important impediment testing, which identifies patients with an IgE-mediated to the care of cancer patients as they may entail serious sensitivity, appears as a promising diagnostic and risk consequences and prevent patients from being treated with stratification tool in the management of patients with the most efficacious agent against their cancer [1]. During the HSRs to taxanes. The management of patients following last decade, different groups have developed rapid drug de- a HSR involves risk stratification and re-exposure could sensitization (RDD) protocols that allow the safe re- be performed either through rapid drug desensitization introduction of a chemotherapeutic agent to which a patient or graded challenge based on the severity of the initial is allergic, and their use have recently been endorsed by the HSR and the skin test result. -

Antiproliferative Effects of Free and Encapsulated Hypericum Perforatum L
ISSN 2093-6966 [Print], ISSN 2234-6856 [Online] Journal of Pharmacopuncture 2019;22[2]:102-108 DOI: https://doi.org/10.3831/KPI.2019.22.013 Original article Antiproliferative Effects of Free and Encapsulated Hypericum Perforatum L. Extract and Its Potential Interaction with Doxorubicin for Esophageal Squamous Cell Carcinoma Issa Amjadi1, Mohammad Mohajeri2, Andrei Borisov3 , Motahare-Sadat Hosseini4* 1 Department of Biomedical Engineering, Wayne State University, Detroit, United States 2 Department of Medical Biotechnology, Mashhad University of Medical Sciences, Mashhad, Iran 3 Department of Biomedical Engineering, Wayne State University, Detroit, United States 4 Biomaterials Group, Department of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran Key Words Results: Free drugs and nanoparticles significantly inhibited KYSE30 cells by 55-73% and slightly affected doxorubicin, hypericum perforatum extract, nano- normal cells up to 29%. The IC50 of Dox NPs and HP particles, esophageal squamous cell carcinoma, drug NPs was ~ 0.04-0.06 mg/mL and ~ 0.6-0.7 mg/mL, re- resistance spectively. Significant decrease occurred in cyclin D1 expression by Dox NPs and HP NPs (P < 0.05). Expo- Abstract sure of KYSE-30 cells to combined treatments includ- ing both Dox and HP extract significantly increased the Objectives: Esophageal squamous cell carcinoma level of cyclin D1 expression as compared to those with (ESCC) is considered as a deadly medical condition that individual treatments (P < 0.05). affects a growing number of people worldwide. Target- ed therapy of ESCC has been suggested recently and Conclusion: Dox NPs and HP NPs can successfully and required extensive research. With cyclin D1 as a thera- specifically target ESCC cells through downregulation peutic target, the present study aimed at evaluating the of cyclin D1. -

Hydroxyacetonitrile (HOCH2CN) As a Precursor for Formylcyanide (CHOCN), Ketenimine (CH2CNH), and Cyanogen (NCCN) in Astrophysical Conditions
A&A 549, A93 (2013) Astronomy DOI: 10.1051/0004-6361/201219779 & c ESO 2013 Astrophysics Hydroxyacetonitrile (HOCH2CN) as a precursor for formylcyanide (CHOCN), ketenimine (CH2CNH), and cyanogen (NCCN) in astrophysical conditions G. Danger1, F. Duvernay1, P. Theulé1, F. Borget1, J.-C. Guillemin2, and T. Chiavassa1 1 Aix-Marseille Univ, CNRS, PIIM UMR 7345, 13397 Marseille, France e-mail: [email protected] 2 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, Avenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France Received 8 June 2012 / Accepted 19 November 2012 ABSTRACT Context. The reactivity in astrophysical environments can be investigated in the laboratory through experimental simulations, which provide understanding of the formation of specific molecules detected in the solid phase or in the gas phase of these environments. In this context, the most complex molecules are generally suggested to form at the surface of interstellar grains and to be released into the gas phase through thermal or non-thermal desorption, where they can be detected through rotational spectroscopy. Here, we focus our experiments on the photochemistry of hydroxyacetonitrile (HOCH2CN), whose formation has been shown to compete with aminomethanol (NH2CH2OH), a glycine precursor, through the Strecker synthesis. Aims. We present the first experimental investigation of the ultraviolet (UV) photochemistry of hydroxyacetonitrile (HOCH2CN) as a pure solid or diluted in water ice. Methods. We used Fourier transform infrared (FT-IR) spectroscopy to characterize photoproducts of hydroxyacetonitrile (HOCH2CN) and to determine the different photodegradation pathways of this compound. To improve the photoproduct identifications, irradiations of hydroxyacetonitrile 14N and 15N isotopologues were performed, coupled with theoretical calculations. -

Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes
Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes May 2013 Authors Mark Ferrey Contributors/acknowledgements The MPCA is reducing printing and mailing costs This report contains the results of a study that by using the Internet to distribute reports and characterizes the presence of unregulated information to wider audience. Visit our website contaminants in Minnesota’s lakes. The study for more information. was made possible through funding by the MPCA reports are printed on 100 percent post- Minnesota Clean Water Fund and by funding by consumer recycled content paper manufactured the U.S. Environmental Protection Agency without chlorine or chlorine derivatives. (EPA), which facilitated the sampling of lakes for this study. The Minnesota Pollution Control Agency (MPCA) thanks the following for assistance and advice in designing and carrying out this study: Steve Heiskary, Pam Anderson, Dereck Richter, Lee Engel, Amy Garcia, Will Long, Jesse Anderson, Ben Larson, and Kelly O’Hara for the long hours of sampling for this study. Cynthia Tomey, Kirsten Anderson, and Richard Grace of Axys Analytical Labs for the expert help in developing the list of analytes for this study and logistics to make it a success. Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | www.pca.state.mn.us | 651-296-6300 Toll free 800-657-3864 | TTY 651-282-5332 This report is available in alternative formats upon request, and online at www.pca.state.mn.us. Document number: tdr-g1-16 Contents Contents ...........................................................................................................................................