Elliotcrespi2009.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Beaver's Phylogenetic Lineage Illuminated by Retroposon Reads
www.nature.com/scientificreports OPEN The Beaver’s Phylogenetic Lineage Illuminated by Retroposon Reads Liliya Doronina1,*, Andreas Matzke1,*, Gennady Churakov1,2, Monika Stoll3, Andreas Huge3 & Jürgen Schmitz1 Received: 13 October 2016 Solving problematic phylogenetic relationships often requires high quality genome data. However, Accepted: 25 January 2017 for many organisms such data are still not available. Among rodents, the phylogenetic position of the Published: 03 March 2017 beaver has always attracted special interest. The arrangement of the beaver’s masseter (jaw-closer) muscle once suggested a strong affinity to some sciurid rodents (e.g., squirrels), placing them in the Sciuromorpha suborder. Modern molecular data, however, suggested a closer relationship of beaver to the representatives of the mouse-related clade, but significant data from virtually homoplasy- free markers (for example retroposon insertions) for the exact position of the beaver have not been available. We derived a gross genome assembly from deposited genomic Illumina paired-end reads and extracted thousands of potential phylogenetically informative retroposon markers using the new bioinformatics coordinate extractor fastCOEX, enabling us to evaluate different hypotheses for the phylogenetic position of the beaver. Comparative results provided significant support for a clear relationship between beavers (Castoridae) and kangaroo rat-related species (Geomyoidea) (p < 0.0015, six markers, no conflicting data) within a significantly supported mouse-related clade (including Myodonta, Anomaluromorpha, and Castorimorpha) (p < 0.0015, six markers, no conflicting data). Most of an organism’s phylogenetic history is fossilized in their heritable genomic material. Using data from genome sequencing projects, particularly informative regions of this material can be extracted in sufficient num- bers to resolve the deepest history of speciation. -

An Exemplary Case Study Gérard Dubost
Convergence characteristics between a rodent, the South American lowland paca, and a ruminant, the African water chevrotain: An exemplary case study Gérard Dubost To cite this version: Gérard Dubost. Convergence characteristics between a rodent, the South American lowland paca, and a ruminant, the African water chevrotain: An exemplary case study. Comptes Rendus Biologies, Elsevier Masson, 2017, 10.1016/j.crvi.2017.02.001. hal-01485153 HAL Id: hal-01485153 https://hal.sorbonne-universite.fr/hal-01485153 Submitted on 8 Mar 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License G Model CRASS3-3495; No. of Pages 10 C. R. Biologies xxx (2017) xxx–xxx Contents lists available at ScienceDirect Comptes Rendus Biologies ww w.sciencedirect.com Ecology/E´ cologie Convergence characteristics between a rodent, the South American lowland paca, and a ruminant, the African water chevrotain: An exemplary case study Caracte`res convergents entre un rongeur, le -

Eocene-Recent Suborder 3 Ruminantia Eocene-Recent I
Order Artiodaetyla Suborder 1 Palaeodonta (ancient teeth) Suborder 2 Suina (Suriformes) Eocene-Recent Suborder 3 Ruminantia Eocene-Recent I p9ojlh Common Wild Boar (Sus scrofa) dgdh&l~tXXhUlla79 %il 21U ‘- ZO411(S) ' 323 pJt 10-136 n$h sus scrofa Jdi lo-137 The wart-hog, Phacochoerus. (From photographs.) 324 add 10-138 Hippopotamus, Hippopofamus. (From photographs.) 20411(S) 325 , Jdd lo-141 Llama, Loma. (From photographs.) 326 ZO41!(S) ZO411(5) 327 Family Cervidae %&319 zo 41 l(S) 329 dd?l lo-147 li%Ei& Cervus rchomburgki 330 zo 41 l(S) 1 pJi4 lo-148 naidi ~Cervus unicolor) Family Giraffidae \ 332 *. zo 41 l(S) ZO411(S) 333 334 ZO411(S) Jd’$ lo-149 Giraffe (Giraffa). (From phorograpb , ddVI lo-151 Pronghorn (Antilocapra up.) Family Bovidae j¶k 10-l& WdlLldl (Bubalus bubalis) 336 zo 41 l(S) 20411(S) 337 338 20411(s) $4 lo-156 Musk-ox i. adi IO-158 Central Asian Yak, &IS (From photographs.) 340 zo 41 l(S) , J¶lwd lo-160 &l841 (Capricornis sumatrensis) 342 zo 411(S) I& lo-161 ll?lJtil (Nemorhaedus griseus) 20411(S) 343 344 ZO411(3 $4 lo-162 fJ&W, UlXllfJ (Nyeticebus cougang) $4 lo-163 Bush-bab,,v ~a,a~o. (From photouapb.) 345 ZO411(S) jdwd lo-164 Ring-tail lemur, Lemur. (From life.) Aye-Aye, Daubentonia. (From a photograph.) 346 20 411(S) JP~; 10-186 Spectral tarsier, Tarsius. (From life.) Suborder 2 Anthropoidea Oligocene-Recent Family Callitrichidae Recent &iaoKhS !&fl 89 marmoset ~dhN8lltl~lUfl~~~flfl Ml9fJ1~MUl i claw dlllU?lt~U~Utt~n premolar 3 molar 2 yolksac 2-3 $a 01w13vb-i erabY wasCaiaa8n “1 w~luob.iTni%d zo 41 I(S) 347 qlk lo-167 Common marmoset, Callithrrw. -

Sequence Variation of Necdin Gene in Bovidae Sunday O
Peters et al. Journal of Animal Science and Technology (2018) 60:32 https://doi.org/10.1186/s40781-018-0191-7 RESEARCH Open Access Sequence variation of necdin gene in Bovidae Sunday O. Peters1*†, Marcos De Donato2*†, Tanveer Hussain3, Hectorina Rodulfo2, Masroor E. Babar3 and Ikhide G. Imumorin4,5 Abstract Background: Necdin (NDN), a member of the melanoma antigen family showing imprinted pattern of expression, has been implicated as causing Prader-Willi symptoms, and known to participate in cellular growth, cellular migration and differentiation. The region where NDN is located has been associated to QTLs affecting reproduction and early growth in cattle, but location and functional analysis of the molecular mechanisms have not been established. Methods: Herewereportthesequencevariationoftheentirecodingsequencefrom72samplesofcattle, yak, buffalo, goat and sheep, and discuss its variation in Bovidae. Median-joining network analysis was used to analyze the variation found in the species. Synonymous and non-synonymous substitution rates were determined for the analysis of all the polymorphic sites. Phylogenetic analysis were carried out among the species of Bovidae to reconstruct their relationships. Results: From the phylogenetic analysis with the consensus sequences of the studied Bovidae species, we found that only 11 of the 26 nucleotide changes that differentiate them produced amino acid changes. All the SNPs found in the cattle breeds were novel and showed similar percentages of nucleotides with non-synonymous substitutions at the N- terminal, MHD and C-terminal (12.3, 12.8 and 12.5%, respectively), and were much higher than the percentage of synonymous substitutions (2.5, 2.6 and 4.9%, respectively). Three mutations in cattle and one in sheep, detected in heterozygous individuals were predicted to be deleterious. -

Phylogeny, Biogeography and Systematic Revision of Plain Long-Nosed Squirrels (Genus Dremomys, Nannosciurinae) Q ⇑ Melissa T.R
Molecular Phylogenetics and Evolution 94 (2016) 752–764 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeny, biogeography and systematic revision of plain long-nosed squirrels (genus Dremomys, Nannosciurinae) q ⇑ Melissa T.R. Hawkins a,b,c,d, , Kristofer M. Helgen b, Jesus E. Maldonado a,b, Larry L. Rockwood e, Mirian T.N. Tsuchiya a,b,d, Jennifer A. Leonard c a Smithsonian Conservation Biology Institute, Center for Conservation and Evolutionary Genetics, National Zoological Park, Washington DC 20008, USA b Division of Mammals, National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington DC 20013-7012, USA c Estación Biológica de Doñana (EBD-CSIC), Conservation and Evolutionary Genetics Group, Avda. Americo Vespucio s/n, Sevilla 41092, Spain d George Mason University, Department of Environmental Science and Policy, 4400 University Drive, Fairfax, VA 20030, USA e George Mason University, Department of Biology, 4400 University Drive, Fairfax, VA 20030, USA article info abstract Article history: The plain long-nosed squirrels, genus Dremomys, are high elevation species in East and Southeast Asia. Received 25 March 2015 Here we present a complete molecular phylogeny for the genus based on nuclear and mitochondrial Revised 19 October 2015 DNA sequences. Concatenated mitochondrial and nuclear gene trees were constructed to determine Accepted 20 October 2015 the tree topology, and date the tree. All speciation events within the plain-long nosed squirrels (genus Available online 31 October 2015 Dremomys) were ancient (dated to the Pliocene or Miocene), and averaged older than many speciation events in the related Sunda squirrels, genus Sundasciurus. -

Chromosomal Evolution in Tenrecs (Microgale and Oryzorictes, Tenrecidae) from the Central Highlands of Madagascar
Chromosome Research (2007) 15:1075–1091 # Springer 2007 DOI: 10.1007/s10577-007-1182-6 Chromosomal evolution in tenrecs (Microgale and Oryzorictes, Tenrecidae) from the Central Highlands of Madagascar C. Gilbert1, S. M. Goodman2,3, V. Soarimalala3,4, L. E. Olson5,P.C.M.O_Brien6, F. F. B. Elder7, F. Yang8, M. A. Ferguson-Smith6 & T. J. Robinson1* 1Evolutionary Genomics Group, Department of Botany and Zoology, University of Stellenbosch, Stellenbosch, South Africa; Tel: +27-21-8083955; Fax: +27-21-8082405; E-mail: [email protected]; 2Department of Zoology, Field Museum of Natural History, Lake Shore Drive, Chicago, IL, USA; 3Vahatra, BP 738, Antananarivo (101), Madagascar; 4De´partement de Biologie Animale, Universite´ d_Antananarivo, BP 906, Antananarivo (101), Madagascar; 5University of Alaska Museum, University of Alaska Fairbanks, Fairbanks, AK, USA; 6Centre for Veterinary Science, University of Cambridge, Cambridge, UK; 7Department of Pathology, Cytogenetics Laboratory, UT Southwestern Medical Center, Dallas, TX, USA; 8The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, UK *Correspondence Received 13 August 2007. Received in revised form and accepted for publication by Pat Heslop-Harrison 2 October 2007 Key words: Afrotheria, cytogenetics, evolution, speciation, Tenrecidae Abstract Tenrecs (Tenrecidae) are a widely diversified assemblage of small eutherian mammals that occur in Madagascar and Western and Central Africa. With the exception of a few early karyotypic descriptions based on conventional staining, nothing is known about the chromosomal evolution of this family. We present a detailed analysis of G-banded and molecularly defined chromosomes based on fluorescence in situ hybridization (FISH) that allows a comprehensive comparison between the karyotypes of 11 species of two closely related Malagasy genera, Microgale (10 species) and Oryzorictes (one species), of the subfamily Oryzorictinae. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Tanganyika Wildlife Park L L C Customer ID: 324586 1037 So 183Rd St W Certificate: 48-C-0156 GODDARD, KS 67052 Site: 001 Tanganyika Wildlife Park L L C
United States Department of Agriculture MSHAVER Animal and Plant Health Inspection Service 2016090000638385 Insp_id Inspection Report Tanganyika Wildlife Park L L C Customer ID: 324586 1037 So 183rd St W Certificate: 48-C-0156 GODDARD, KS 67052 Site: 001 Tanganyika Wildlife Park L L C Type: ROUTINE INSPECTION Date: 21-JUN-2021 2.131(b)(1) Critical Handling of animals. This licensee made available for transport to another licensed facility three Eurasian Lynx kittens under 28 days of age. The Certificate of Veterinary Inspection (CVI) shows an inspection date of 5/4/2020 with the kittens at 18 days of age. The shipping date on the CVI matches the APHIS 7020 form provided by the licensee showing a transport date of 5/6/2020. Neonatal nondomestic cats have special handing and husbandry needs and are placed in danger when they are exposed to members of the public and/or stressful conditions, including transportation. Cubs under 4 weeks of age (28 days) do not have a developed immune system, are susceptible to a wide variety of diseases including those of domestic cats, and cannot thermoregulate. The licensee must ensure that handling of all animals be done as expeditiously and carefully as possible in a manner that does not cause trauma, overheating, excessive cooling, behavioral stress, phy sical harm, or unnecessary discomfort. To be corrected from this day forward. This inspection and exit interview were conducted with facility representatives. Additional Inspectors: MICHAEL TYGART, VETERINARY MEDICAL OFFICEREnd Section Prepared By: MARGARET -
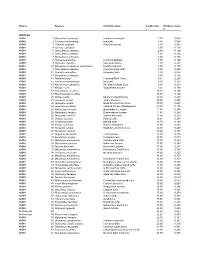
PDF File Containing Table of Lengths and Thicknesses of Turtle Shells And
Source Species Common name length (cm) thickness (cm) L t TURTLES AMNH 1 Sternotherus odoratus common musk turtle 2.30 0.089 AMNH 2 Clemmys muhlenbergi bug turtle 3.80 0.069 AMNH 3 Chersina angulata Angulate tortoise 3.90 0.050 AMNH 4 Testudo carbonera 6.97 0.130 AMNH 5 Sternotherus oderatus 6.99 0.160 AMNH 6 Sternotherus oderatus 7.00 0.165 AMNH 7 Sternotherus oderatus 7.00 0.165 AMNH 8 Homopus areolatus Common padloper 7.95 0.100 AMNH 9 Homopus signatus Speckled tortoise 7.98 0.231 AMNH 10 Kinosternon subrabum steinochneri Florida mud turtle 8.90 0.178 AMNH 11 Sternotherus oderatus Common musk turtle 8.98 0.290 AMNH 12 Chelydra serpentina Snapping turtle 8.98 0.076 AMNH 13 Sternotherus oderatus 9.00 0.168 AMNH 14 Hardella thurgi Crowned River Turtle 9.04 0.263 AMNH 15 Clemmys muhlenbergii Bog turtle 9.09 0.231 AMNH 16 Kinosternon subrubrum The Eastern Mud Turtle 9.10 0.253 AMNH 17 Kinixys crosa hinged-back tortoise 9.34 0.160 AMNH 18 Peamobates oculifers 10.17 0.140 AMNH 19 Peammobates oculifera 10.27 0.140 AMNH 20 Kinixys spekii Speke's hinged tortoise 10.30 0.201 AMNH 21 Terrapene ornata ornate box turtle 10.30 0.406 AMNH 22 Terrapene ornata North American box turtle 10.76 0.257 AMNH 23 Geochelone radiata radiated tortoise (Madagascar) 10.80 0.155 AMNH 24 Malaclemys terrapin diamondback terrapin 11.40 0.295 AMNH 25 Malaclemys terrapin Diamondback terrapin 11.58 0.264 AMNH 26 Terrapene carolina eastern box turtle 11.80 0.259 AMNH 27 Chrysemys picta Painted turtle 12.21 0.267 AMNH 28 Chrysemys picta painted turtle 12.70 0.168 AMNH 29 -

The Ethmoidal Region of the Skull of Ptilocercus Lowii
Research Article Primate Biol., 2, 89–110, 2015 www.primate-biol.net/2/89/2015/ doi:10.5194/pb-2-89-2015 © Author(s) 2015. CC Attribution 3.0 License. The ethmoidal region of the skull of Ptilocercus lowii (Ptilocercidae, Scandentia, Mammalia) – a contribution to the reconstruction of the cranial morphotype of primates I. Ruf1, S. Janßen2, and U. Zeller2 1Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Abteilung Paläoanthropologie und Messelforschung, Senckenberganlage 25, 60325 Frankfurt am Main, Germany 2FG Spezielle Zoologie, Lebenswissenschaftliche Fakultät, Albrecht Daniel Thaer-Institut für Agrar- und Gartenbauwissenschaften, Humboldt-Universität zu Berlin, Ziegelstrasse 5–9, 10117 Berlin, Germany Dedicated to Hans-Jürg Kuhn on the occasion of his 80th birthday. Correspondence to: I. Ruf ([email protected]) Received: 17 June 2015 – Revised: 6 September 2015 – Accepted: 7 September 2015 – Published: 25 September 2015 Abstract. The ethmoidal region of the skull houses one of the most important sense organs of mammals, the sense of smell. Investigation of the ontogeny and comparative anatomy of internal nasal structures of the macros- matic order Scandentia is a significant contribution to the understanding of the morphotype of Scandentia with potential implications for our understanding of the primate nasal morphological pattern. For the first time peri- natal and adult stages of Ptilocercus lowii and selected Tupaia species were investigated by serial histological sections and high-resolution computed tomography (µCT), respectively. Scandentia show a very common olfac- tory turbinal pattern of small mammals in having two frontoturbinals, three ethmoturbinals, and one interturbinal between the first and second ethmoturbinal. This indicates a moderately developed sense of smell (moderately macrosmatic). -

(Mammalia) from the Eocene of Black Crow, Namibia Martin PICKFORD
Tiny Tenrecomorpha (Mammalia) from the Eocene of Black Crow, Namibia Martin PICKFORD Sorbonne Universités (CR2P, MNHN, CNRS, UPMC - Paris VI) 8, rue Buffon, 75005, Paris, France, (e-mail : [email protected]) Abstract: The 2019 campaign of acid treatment of Eocene freshwater limestone from Black Crow, Namibia, resulted in the recovery of a minuscule mandible of an insectivoran-grade mammal representing a new genus and species of Tenrecomorpha. The specimen is the smallest mammal described from the fossil record from Africa. From the incisor alveoli to the rear end of the angle, the jaw measures a mere 8.6 mm. The jaw is relatively complete, but has lost the incisors, canine and p/2. It shows several characters that link it to the suborder Tenrecomorpha. In some morphological features it recalls Tenrecidae, in others Potamogalidae. The new genus and species throws doubt on the homogeneity of the order Afroinsectiphilia, which in its turn renders doubtful the concept of Afrotheria as currently understood. Key words: Tenrec, Ypresian/Lutetian, Mandible, Namibia To cite this paper: Pickford, M. 2019. Tiny Tenrecomorpha (Mammalia) from the Eocene of Black Crow, Namibia. Communications of the Geological Survey of Namibia, 21, 15-25. Introduction This paper is devoted to the description and Each year the Namibia Palaeontology interpretation of a minuscule mammalian Expedition has visited the locality to search for mandible from the middle Eocene limestones at blocks of limestone showing the presence of Black Crow, Namibia. The freshwater bones and teeth on their surfaces and these have limestone at Black Crow in the Sperrgebiet, been developed in the laboratory to extract the Namibia, first yielded vertebrate fossils a fossils. -

Morphological Diversity in Tenrecs (Afrosoricida, Tenrecidae)
Morphological diversity in tenrecs (Afrosoricida, Tenrecidae): comparing tenrec skull diversity to their closest relatives Sive Finlay and Natalie Cooper School of Natural Sciences, Trinity College Dublin, Dublin, Ireland Trinity Centre for Biodiversity Research, Trinity College Dublin, Dublin, Ireland ABSTRACT It is important to quantify patterns of morphological diversity to enhance our un- derstanding of variation in ecological and evolutionary traits. Here, we present a quantitative analysis of morphological diversity in a family of small mammals, the tenrecs (Afrosoricida, Tenrecidae). Tenrecs are often cited as an example of an ex- ceptionally morphologically diverse group. However, this assumption has not been tested quantitatively. We use geometric morphometric analyses of skull shape to test whether tenrecs are more morphologically diverse than their closest relatives, the golden moles (Afrosoricida, Chrysochloridae). Tenrecs occupy a wider range of ecological niches than golden moles so we predict that they will be more morpho- logically diverse. Contrary to our expectations, we find that tenrec skulls are only more morphologically diverse than golden moles when measured in lateral view. Furthermore, similarities among the species-rich Microgale tenrec genus appear to mask higher morphological diversity in the rest of the family. These results reveal new insights into the morphological diversity of tenrecs and highlight the impor- tance of using quantitative methods to test qualitative assumptions about patterns of morphological diversity. Submitted 29 January 2015 Subjects Evolutionary Studies, Zoology Accepted 13 April 2015 Keywords Golden moles, Geometric morphometrics, Disparity, Morphology Published 30 April 2015 Corresponding author Natalie Cooper, [email protected] INTRODUCTION Academic editor Analysing patterns of morphological diversity (the variation in physical form Foote, Laura Wilson 1997) has important implications for our understanding of ecological and evolutionary Additional Information and traits.