Kidneys of Mice with Hereditary Tyrosinemia Type I Are Extremely Sensitive to Cytotoxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newborn Screening Laboratory Manual of Services
Newborn Screening Laboratory Manual of Services Test Panel: Please see the following links for a detailed description of testing in the Newborn Screening section. Information about the Newborn Screening program is available here. Endocrine Disorders Congenital adrenal hyperplasia (CAH) Congenital hypothyroidism (TSH) Hemoglobinopathies Sickle cell disease (FS) Alpha (Barts) Sickle βeta Thalassemia (FSA) Other sickling hemoglobinopathies such as: FAS FAC FAD FAE Homozygous conditions such as: FC FD FE Metabolic Disorders Biotinidase deficiency Galactosemia Cystic fibrosis (CF) first tier screening for elevated immunoreactive trypsinogen (IRT) Cystic fibrosis second tier genetic mutation analysis on the top 4% IRT concentrations. Current alleles detected : F508del, I507del, G542X, G85E, R117H, 621+1G->T, 711+1G->T, R334W, R347P, A455E, 1717-1G->A, R560T, R553X, G551D, 1898+1G->A, 2184delA, 2789+5G->A, 3120+1G->A, R1162X, 3659delC, 3849+10kbC->T, W1282X, N1303K, IVS polyT T5/T7/T9 *Currently validating a mutation panel that includes the above alleles in addition to the following: 1078delT, Y122X, 394delTT, R347H, M1101K, S1255X, 1898+5G->T, 2183AA->G, 2307insA, Y1092X, 3876delA, 3905insT, S549N, S549R_1645A->C, S549R-1647T->G, S549R-1647T->G, V520F, A559T, 1677delTA, 2055del9->A, 2143delT, 3199del6, 406-1G->A, 935delA, D1152H, CFTRdele2, E60X, G178R, G330X, K710X, L206W, Q493X, Q890X, R1066C, R1158X, R75X, S1196X, W1089X, G1244E, G1349D, G551S, R560KT, S1251N, S1255P Amino acid disorders Phenylketonuria (PKU) / Hyperphenylalaninemia Maple -

TYR I, II, III Act Sheet
Newborn Screening ACT Sheet Increased Tyrosine Tyrosinemia Differential Diagnosis: Tyrosinemia I (hepatorenal); Tyrosinemia II (oculocutaneous); Tyrosinemia III; transient hypertyrosinemia; liver disease. Condition Description: In the hepatorenal form, tyrosine from ingested protein and phenylalanine metabolism cannot be metabolized by fumarylacetoacetate hydrolase to fumaric acid and acetoacetic acid. The resulting fumarylacetoacetate accumulates and is converted to succinylacetone, the diagnostic metabolite, which is liver toxic, and leads to elevated tyrosine. Tyrosinemias II and III are due to other defects in tyrosine degradation. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: plasma quantitative amino acids; urine succinylacetone and liver function tests. • Repeat newborn screen if the second screen has not been done. • Provide family with basic information about tyrosinemia. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino acid analysis will show increased tyrosine in all of the tyrosinemias. Urine organic acid analysis may reveal increased succinylacetone in Tyrosinemia I. Clinical Considerations: Tyrosinemia I is usually asymptomatic in the neonate. If untreated, it will cause liver disease and cirrhosis -
Download This PDF File
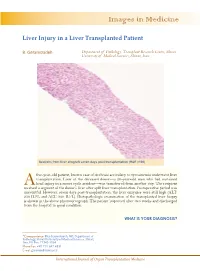
Images in Medicine Liver Injury in a Liver Transplanted Patient B. Geramizadeh Department of Pathology, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Sections from liver allograft seven days post-transplantation (H&E ×100) five-year-old patient, known case of cirrhosis secondary to tyrosinemia underwent liver transplantation. Liver of the deceased donor—a 20-year-old man who had sustained A head injury in a motor cycle accident—was transferred from another city. The recipient received a segment of the donor’s liver after split liver transplantation. Postoperative period was uneventful. However, seven days post-transplantation, the liver enzymes were still high (ALT: 250 IU/L and ALT: 320 IU/L). Histopathologic examination of the transplanted liver biopsy is shown in the above photomicrograph. The patient improved after two weeks and discharged from the hospital in good condition. WHAT IS YOUR DIAGNOSIS? *Correspondence: Bita Geramizadeh, MD, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran. PO Box: 71345-1864 Phone/Fax: +98-711-647-4331 E-mail: [email protected] International Journal of Organ Transplantation Medicine B. Geramizadeh DIAGNOSIS: PRESERVATION/REPERFUSION INJURY dvances in organ preservation have duct obstruction. The distinctive pattern of reduced preservation injury. Neverthe- bile ductular cholestasis is that it is usually as- Aless, when storage time exceeds 10 to sociated with sepsis. Drug toxicity can mimic 12 hours, post-transplantation complications, every change in the liver and should always be due to preservation/reperfusion injury, be- excluded [5]. come more common [1]. In our patient, prolonged cold ischemic time Ischemic injury to the graft is divided into (transfer of the liver from another city) and cold ischemia—secondary to prolonged pres- probably small for size graft (split liver trans- ervation—and warm ischemia, which occurs plantation) were predisposing factors. -

What Disorders Are Screened for by the Newborn Screen?
What disorders are screened for by the newborn screen? Endocrine Disorders The endocrine system is important to regulate the hormones in our bodies. Hormones are special signals sent to various parts of the body. They control many things such as growth and development. The goal of newborn screening is to identify these babies early so that treatment can be started to keep them healthy. To learn more about these specific disorders please click on the name of the disorder below: English: Congenital Adrenal Hyperplasia Esapnol Hiperplasia Suprarrenal Congenital - - http://www.newbornscreening.info/Parents/otherdisorders/CAH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CAH.html - Congenital Hypothyroidism (Hipotiroidismo Congénito) - http://www.newbornscreening.info/Parents/otherdisorders/CH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CH.html Hematologic Conditions Hemoglobin is a special part of our red blood cells. It is important for carrying oxygen to the parts of the body where it is needed. When people have problems with their hemoglobin they can have intense pain, and they often get sick more than other children. Over time, the lack of oxygen to the body can cause damage to the organs. The goal of newborn screening is to identify babies with these conditions so that they can get early treatment to help keep them healthy. To learn more about these specific disorders click here (XXX). - Sickle Cell Anemia (Anemia de Célula Falciforme) - http://www.newbornscreening.info/Parents/otherdisorders/SCD.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/SCD.html - SC Disease (See Previous Link) - Sickle Beta Thalassemia (See Previous Link) Enzyme Deficiencies Enzymes are special proteins in our body that allow for chemical reactions to take place. -

Tyrosinemia (Type I) – Amino Acid Disorder
Tyrosinemia (Type I) – Amino Acid Disorder What are amino acid disorders? Kidney disease may lead to rickets, a bone The amino acid disorders are a class of disease. The nerves may also be affected. inherited metabolic conditions that occur when Some babies may have a rapid heart rate, certain amino acids either cannot be broken breathing difficulties, and seizures. down or cannot be produced by the body, Occasionally, individuals with liver damage resulting in the toxic accumulation of some have a higher risk of developing liver cancer. substances and the deficiency of other Acute liver and kidney damage can lead to substances. death. What is tyrosinemia? How is the diagnosis confirmed? In tyrosinemia, the amino acid tyrosine cannot The diagnosis is confirmed by measuring the be broken down properly, leading to a toxic levels of amino acids in the blood and organic accumulation of this amino acid and its acids in the urine. The finding of metabolites in the body. succinylacetone in the urine is diagnostic. Enzyme testing and genetic testing of the FAH What is its incidence? gene may also be used to confirm the Tyrosinemia affects about 1 out every 100,000 diagnosis. Diagnostic testing is arranged by babies born in BC. Although tyrosinemia specialists at BC Children’s Hospital. occurs in all ethnic groups, it is more common in certain populations. Its incidence has been What is the treatment of the disease? reported as high as 1 in 2,000 in the French Children with tyrosinemia are treated with a Canadian population living in the Saguenay- medication called nitisinone (previously called Lac-St-Jean region of Quebec. -

Disorders Alphabetical by Disease Updated 1/2020
Disorders Alphabetical by Disease updated 1/2020 Disorders Abbreviation Classification Recommended Uniform Screening Panel (RUSP) Classification 2,4 Dienoyl CoA Reductase Deficiency DE RED Fatty Acid Oxidation Disorder Secondary Condition 2-Methyl 3 Hydroxy Butyric Aciduria 2M3HBA Organic Acid Disorder Secondary Condition 2-Methyl Butyryl-CoA Dehydrogenase Deficiency 2MBG Organic Acid Disorder Secondary Condition (called 2-Methylbutyrylglycinuria on RUSP) 3-Hydroxy-3-Methylglutaryl CoA Lyase Deficiency HMG Organic Acid Disorder Core Condition 3-Methylcrotonyl CoA Carboxylase Deficiency 3MCC Organic Acid Disorder Core Condition 3-Methylglutaconic Aciduria 3MGA Organic Acid Disorder Secondary Condition Alpha-Thalassemia (Bart's Hb) Hemoglobin Bart's Hemoglobin Disorder Secondary Conditoin Argininemia, Arginase Deficiency ARG Amino Acid Disorder Secondary Condition Arginosuccinic Aciduria ASA Amino Acid Disorder Core Condition Benign Hyperphenylalaninemia PHE Amino Acid Disorder Secondary Condition Beta-Ketothiolase Deficiency BKT Organic Acid Disorder Core Condition Biopterin Defect in Cofactor Biosynthesis BIOPT (BS) Amino Acid Disorder Secondary Condition Biopterin Defect in Cofactor Regeneration BIOPT (Reg) Amino Acid Disorder Secondary Condition Biotinidase Deficiency BIO Metabolic Disorder of Biotin Recycling Core Condition Carbamoyltransferase Deficiency, Carbamoyl Phosphate Synthetase I Deficiency CPS Amino Acid Disorder Not on RUSP Carnitine Palmitoyl Transferase Deficiency Type 1 CPT I Fatty Acid Oxidation Disorder Secondary Condition -

And L-2-Hydroxyglutaric Acid: Application to the ~Etectionand Prenatal Diagnosis of D- and L-2-Hydroxyglutaric Acidemias
003 1-3998/93/3403-0277$03.00/0 PEDIATRIC RESEARCH Vol. 34. No. 3. 1993 Copyright 0 1993 International Pediatric Research Foundation, Inc. Prinrc.d in U.S. A. Stable-Isotope Dilution Analysis of D- and L-2-Hydroxyglutaric Acid: Application to the ~etectionand Prenatal Diagnosis of D- and L-2-Hydroxyglutaric Acidemias K. M. GIBSON', H. J. TEN BRINK, D. S. M. SCHOR. R. M. KOK, A. H. BOOTSMA. G. F. HOFFMANN, AND C. JAKOBS D~parrt~lcnrofPc9diutric~, Free Univcrsit!~Ilospitul, ilt~~srcrdut)~,T11eNc>tlrerlunds /K,hf.G., II.J.T.B., D.S.IZ~.S., R.jZ1.K.. A.11. B.. C.J.],and Department of Pedialrics, Univ~rsityof H~idelberg,Gcrmuny /G.I;.II.] ABSTRACT. A stable-isotope dilution assay has been gation on the concentrations of D- and L-2-HG acids in human developed for quantitation of D- and L-2-hydroxyglutaric physiologic fluids. The separation of D- and L-2-HG acid requires acids in physiologic fluids. D- and L-2-hydroxyglutaric the expensive preparation of chiral derivatives using (D)-2-bu- acids are separated as the 0-acetyl-di-(D)-2-butyl esters. tanol, which may account for the lack of detailed analyses of the The method uses ~,~-[3,3,4,4-~H~]-2-hydroxyglutaricacid different configurations in humans (2, 3). as internal standard with ammonia chemical ionization, Since the description of the index patients with D- and L-2- selected ion monitoring gas chromatography-mass spec- HG aciduria (2, 3), a number of patients with 2-HG aciduria trometry. For 13 patients with L-2-hydroxyglutaric acidu- have been reported. -

Diseases Catalogue
Diseases catalogue AA Disorders of amino acid metabolism OMIM Group of disorders affecting genes that codify proteins involved in the catabolism of amino acids or in the functional maintenance of the different coenzymes. AA Alkaptonuria: homogentisate dioxygenase deficiency 203500 AA Phenylketonuria: phenylalanine hydroxylase (PAH) 261600 AA Defects of tetrahydrobiopterine (BH 4) metabolism: AA 6-Piruvoyl-tetrahydropterin synthase deficiency (PTS) 261640 AA Dihydropteridine reductase deficiency (DHPR) 261630 AA Pterin-carbinolamine dehydratase 126090 AA GTP cyclohydrolase I deficiency (GCH1) (autosomal recessive) 233910 AA GTP cyclohydrolase I deficiency (GCH1) (autosomal dominant): Segawa syndrome 600225 AA Sepiapterin reductase deficiency (SPR) 182125 AA Defects of sulfur amino acid metabolism: AA N(5,10)-methylene-tetrahydrofolate reductase deficiency (MTHFR) 236250 AA Homocystinuria due to cystathionine beta-synthase deficiency (CBS) 236200 AA Methionine adenosyltransferase deficiency 250850 AA Methionine synthase deficiency (MTR, cblG) 250940 AA Methionine synthase reductase deficiency; (MTRR, CblE) 236270 AA Sulfite oxidase deficiency 272300 AA Molybdenum cofactor deficiency: combined deficiency of sulfite oxidase and xanthine oxidase 252150 AA S-adenosylhomocysteine hydrolase deficiency 180960 AA Cystathioninuria 219500 AA Hyperhomocysteinemia 603174 AA Defects of gamma-glutathione cycle: glutathione synthetase deficiency (5-oxo-prolinuria) 266130 AA Defects of histidine metabolism: Histidinemia 235800 AA Defects of lysine and -

Tyrosinemia Type I and Reversible Neurogenic Crisis After a One-Month Interruption of Nitisinone
J Pediatr Res 2018;5(Supple 1):57-9 DO I: 10.4274/jpr42275 Case Report Tyrosinemia Type I and Reversible Neurogenic Crisis After a One-Month Interruption of Nitisinone Havva Yazıcı1, Ebru Canda1, Esra Er1, Mehmet Arda Kılınç2, Sema Kalkan Uçar1, Bülent Karapınar2, Mahmut Çoker1 1Ege University Faculty of Medicine, Department of Pediatrics, Division of Pediatrics Metabolism and Nutrition, İzmir, Turkey 2Ege University Faculty of Medicine, Department of Pediatrics, Division of Pediatric Intensive Care Unit, İzmir, Turkey ABS TRACT Hereditary tyrosinemia Type I (HTI) is an autosomal recessive disorder due to a deficiency of the enzyme fumarylacetoacetate hydrolase. The liver is the primary organ that is affected and comorbidities with renal and neurologic systems and hepatocellular carcinoma can be seen as a long-term complication. An effective treatment has been available with 2-[2-nitro-4-trifluoromethylbenzoyl]-1,3-cyclohexanedione (NTBC) since 1992. Neurogenic crises do not take place in HTI patients who are treated with NTBC. Here, we report on a seven-year-old boy who underwent a severe neurological crisis including anorexia, vomiting, weakness, hyponatremia, paresthesia and paralysis of the extremities, seizure and arterial hypertension after an interruption of NTBC treatment. With the re-introduction of NTBC, the patient gradually reacquired normal neurological functions, normal blood pressure and recovered completely. Keywords: Tyrosinemia Type I, neurogenic crises, nitisinone Introduction effective within hours, eradicating hepatic and neurological findings and protecting from the risk of hepatocellular Hereditary tyrosinemia Type I (HTI) (OMIM 276700) carcinoma when treatment starts within the first months of is a rare inborn error of the tyrosine metabolism due to life (3). -

Transient Tyrosinemia Resolves Within a Month Or Two of Birth Or Vitamin C Supplements for a Few Days Will Shorten the Time
OFFICE OF THE SECRETARY OF STATE ARCHIVES DIVISION BEV CLARNO STEPHANIE CLARK SECRETARY OF STATE INTERIM DIRECTOR A. RICHARD VIAL 800 SUMMER STREET NE DEPUTY SECRETARY OF STATE SALEM, OR 97310 503-373-0701 NOTICE OF PROPOSED RULEMAKING INCLUDING STATEMENT OF NEED & FISCAL IMPACT FILED 08/20/2019 3:26 PM CHAPTER 333 ARCHIVES DIVISION OREGON HEALTH AUTHORITY SECRETARY OF STATE PUBLIC HEALTH DIVISION FILING CAPTION: Update of Newborn Bloodspot Screening rules LAST DAY AND TIME TO OFFER COMMENT TO AGENCY: 09/23/2019 5:00 PM The Agency requests public comment on whether other options should be considered for achieving the rule's substantive goals while reducing negative economic impact of the rule on business. CONTACT: Brittany Hall 800 NE Oregon St. Suite 930 Filed By: 503-449-9808 Portland,OR 97232 Brittany Hall [email protected] Rules Coordinator HEARING(S) Auxilary aids for persons with disabilities are available upon advance request. Notify the contact listed above. DATE: 09/16/2019 TIME: 2:30 PM OFFICER: Staff ADDRESS: Portland State Office Building 800 NE Oregon St. Room 1D Portland, OR 97232 NEED FOR THE RULE(S): The Oregon Health Authority (Authority), Public Health Division, Oregon State Public Health Laboratory's (OSPHL) Northwest Regional Newborn Bloodspot Screening Program (NWRNBS Program) is proposing permanent amendments to administrative rules in chapter 333, division 24 pertaining to newborn screening to update and clarify rules. The proposed rule amendments update the rules regarding the definition of terms used, timing for collecting specimens, methods of testing and the retention of residual specimens. In addition, the proposed rule amendments include housekeeping edits and update the reference to the Oregon Newborn Bloodspot Screening Practitioner’s Manual throughout the rules. -

Texas Newborn Screening Panel
TEXAS NEWBORN SCREENING PANEL BLOODSPOT TESTING (conducted at DSHS Laboratory) Amino Acid Disorders Core Conditions Secondary Conditions • Argininosuccinic Aciduria (ASA) • Argininemia (ARG) • Citrullinemia, Type I (CIT) • Benign Hyperphenylalaninemia (H-PHE) • Homocystinuria (HCY) • Biopterin defect in cofactor biosynthesis (BIOPT BS) • Maple Syrup Urine Disease (MSUD) • Biopterin defect in cofactor regeneration (BIOPT REG) • Classic Phenylketonuria (PKU) • Citrullinemia, Type II (CIT II) • Tyrosinemia, Type I (TYR I) • Hypermethioninemia (MET) • Tyrosinemia, Type II (TYR II) • Tyrosinemia, Type III (TYR III) Fatty Acid Disorders Core Conditions Secondary Conditions • Carnitine Uptake Defect (CUD) • 2,4 Dienoyl-CoA Reductase Deficiency (DE RED) • Long Chain L-3-Hydroxyacyl-CoA Dehydrogenase Deficiency • Carnitine Acylcarnitine Translocase Deficiency (CACT) (LCHAD) • Carnitine Palmitoyltransferase Type I Deficiency (CPT I) • Medium-Chain Acyl-CoA Dehydrogenase Deficiency (MCAD) • Carnitine Palmitoyltransferase Type II Deficiency (CPT II) • Trifunctional Protein Deficiency (TFP) • Glutaric Acidemia Type II (GA2) • Very Long-Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD) • Medium-Chain Ketoacyl-CoA Thiolase Deficiency (MCKAT) • Medium/Short Chain L-3-Hydroxyacyl-CoA Dehydrogenase Deficiency (M/SCHAD) • Short-Chain Acyl-CoA Dehydrogenase Deficiency (SCAD) Organic Acid Disorders Core Conditions Secondary Conditions • 3-Methylcrotonyl-CoA Carboxylase Deficiency (3-MCC) • 2 Methylbutyrylglycinuria (2MBG) • 3-Hydroxy-3-Methylglutaric Aciduria -

Tyrosinemia Information for Physicians and Other Health Care Professionals
NEWBORN SCREENING OFFICE OF HEALTH PROMOTION 535 W. Jefferson St., 2nd Floor Springfield, IL 62761 Phone: 217-785-8101 Fax: 217-557-5396 Tyrosinemia Information for Physicians and Other Health Care Professionals Definition The tyrosinemias are a group of inherited disorders of amino acid metabolism, each caused by an enzymatic defect affecting tyrosine catabolism, which leads to elevated levels of tyrosine. Newborn screening in Illinois includes testing for the following type of tyrosinemia: Tyrosinemia type I (hepatorenal tyrosinemia or fumarylacetoacetate hydrolase (FAH) deficiency) Note: Some cases of tyrosinemia may not be detected by newborn screening when specimens are collected in the first few days of life, as tyrosine levels may not be sufficiently elevated for detection by tandem mass spectrometry. Clinical Symptoms There is variability in age of onset, depending on the type of tyrosinemia. In some forms of the disease, children may be clinically diagnosed in the neonatal period. Type I, the most severe form of tyrosinemia, results in the accumulation of tyrosine and its metabolites in the liver causing severe liver disease. Kidney function and peripheral nerves also are affected. Patients with Type I may have acute liver crisis, episodes of peripheral neuropathy and chronic liver disease. Effects on the kidneys can range from mild tubular dysfunction to renal failure. Early symptoms can include fever, diarrhea, vomiting, enlarged liver, jaundice, rickets, lethargy and irritability. Newborn Screening and Definitive Diagnosis In Illinois, newborn screening for tyrosinemia type I is performed using tandem mass spectrometry. False positive and false negative results may be possible with this screening. Not all cases of tyrosinemia will be detected by newborn screening.