Transient Tyrosinemia Resolves Within a Month Or Two of Birth Or Vitamin C Supplements for a Few Days Will Shorten the Time
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperammonemia in Review: Pathophysiology, Diagnosis, and Treatment
Pediatr Nephrol DOI 10.1007/s00467-011-1838-5 EDUCATIONAL REVIEW Hyperammonemia in review: pathophysiology, diagnosis, and treatment Ari Auron & Patrick D. Brophy Received: 23 September 2010 /Revised: 9 January 2011 /Accepted: 12 January 2011 # IPNA 2011 Abstract Ammonia is an important source of nitrogen and is the breakdown and catabolism of dietary and bodily proteins, required for amino acid synthesis. It is also necessary for respectively. In healthy individuals, amino acids that are not normal acid-base balance. When present in high concentra- needed for protein synthesis are metabolized in various tions, ammonia is toxic. Endogenous ammonia intoxication chemical pathways, with the rest of the nitrogen waste being can occur when there is impaired capacity of the body to converted to urea. Ammonia is important for normal animal excrete nitrogenous waste, as seen with congenital enzymatic acid-base balance. During exercise, ammonia is produced in deficiencies. A variety of environmental causes and medica- skeletal muscle from deamination of adenosine monophos- tions may also lead to ammonia toxicity. Hyperammonemia phate and amino acid catabolism. In the brain, the latter refers to a clinical condition associated with elevated processes plus the activity of glutamate dehydrogenase ammonia levels manifested by a variety of symptoms and mediate ammonia production. After formation of ammonium signs, including significant central nervous system (CNS) from glutamine, α-ketoglutarate, a byproduct, may be abnormalities. Appropriate and timely management requires a degraded to produce two molecules of bicarbonate, which solid understanding of the fundamental pathophysiology, are then available to buffer acids produced by dietary sources. differential diagnosis, and treatment approaches available. -

Newborn Screening Laboratory Manual of Services
Newborn Screening Laboratory Manual of Services Test Panel: Please see the following links for a detailed description of testing in the Newborn Screening section. Information about the Newborn Screening program is available here. Endocrine Disorders Congenital adrenal hyperplasia (CAH) Congenital hypothyroidism (TSH) Hemoglobinopathies Sickle cell disease (FS) Alpha (Barts) Sickle βeta Thalassemia (FSA) Other sickling hemoglobinopathies such as: FAS FAC FAD FAE Homozygous conditions such as: FC FD FE Metabolic Disorders Biotinidase deficiency Galactosemia Cystic fibrosis (CF) first tier screening for elevated immunoreactive trypsinogen (IRT) Cystic fibrosis second tier genetic mutation analysis on the top 4% IRT concentrations. Current alleles detected : F508del, I507del, G542X, G85E, R117H, 621+1G->T, 711+1G->T, R334W, R347P, A455E, 1717-1G->A, R560T, R553X, G551D, 1898+1G->A, 2184delA, 2789+5G->A, 3120+1G->A, R1162X, 3659delC, 3849+10kbC->T, W1282X, N1303K, IVS polyT T5/T7/T9 *Currently validating a mutation panel that includes the above alleles in addition to the following: 1078delT, Y122X, 394delTT, R347H, M1101K, S1255X, 1898+5G->T, 2183AA->G, 2307insA, Y1092X, 3876delA, 3905insT, S549N, S549R_1645A->C, S549R-1647T->G, S549R-1647T->G, V520F, A559T, 1677delTA, 2055del9->A, 2143delT, 3199del6, 406-1G->A, 935delA, D1152H, CFTRdele2, E60X, G178R, G330X, K710X, L206W, Q493X, Q890X, R1066C, R1158X, R75X, S1196X, W1089X, G1244E, G1349D, G551S, R560KT, S1251N, S1255P Amino acid disorders Phenylketonuria (PKU) / Hyperphenylalaninemia Maple -

Disorders Included in the Newborn Screening Panel Disorders
Disorders Included in the Newborn Screening Panel Disorders Detected by Tandem Mass Spectrometry Acylcarnitine Profile Amino Acid Profile Fatty Acid Oxidation Disorders Amino Acid Disorders Carnitine/Acylcarnitine Translocase Deficiency Argininemia 1 Carnitine Palmitoyl Transferase Deficiency Type I Argininosuccinic Aciduria 1 3-Hydroxy Long Chain Acyl-CoA Dehydrogenase 5-Oxoprolinuria 1 Deficiency Carbamoylphosphate Synthetase Deficiency 1 2,4-Dienoyl-CoA Reductase Deficiency Citrullinemia Medium Chain Acyl-CoA Dehydrogenase Deficiency Homocystinuria Multiple Acyl-CoA Dehydrogenase Deficiency Hypermethioninemia Neonatal Carnitine Palmitoyl Transferase Deficiency Hyperammonemia, Hyperornithinemia, Homocitrullinuria Type II Syndrome1 1 Short Chain Acyl-CoA Dehydrogenase Deficiency Hyperornithinemia with Gyral Atrophy Short Chain Hydroxy Acyl-CoA Dehydrogenase Maple Syrup Urine Disease Deficiency Phenylketonuria Trifunctional Protein Deficiency Classical/Hyperphenylalaninemia Very Long Chain Acyl-CoA Dehydrogenase Deficiency Biopterin Cofactor Deficiencies Tyrosinemia Organic Acid Disorders Transient Neonatal Tyrosinemia 2 Tyrosinemia Type I 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency Tyrosinemia Type II Glutaric Acidemia Type I Tyrosinemia Type III Isobutyryl-CoA Dehydrogenase Deficiency Isovaleric Acidemia 2-Methylbutyryl-CoA Dehydrogenase Deficiency 3-Methylcrotonyl-CoA Carboxylase Deficiency Other Observations 3-Methylglutaconyl-CoA Hydratase Deficiency Methylmalonic Acidemias Hyperalimentation Methylmalonyl-CoA Mutase Deficiency -

Incidence of Inborn Errors of Metabolism by Expanded Newborn
Original Article Journal of Inborn Errors of Metabolism & Screening 2016, Volume 4: 1–8 Incidence of Inborn Errors of Metabolism ª The Author(s) 2016 DOI: 10.1177/2326409816669027 by Expanded Newborn Screening iem.sagepub.com in a Mexican Hospital Consuelo Cantu´-Reyna, MD1,2, Luis Manuel Zepeda, MD1,2, Rene´ Montemayor, MD3, Santiago Benavides, MD3, Hector´ Javier Gonza´lez, MD3, Mercedes Va´zquez-Cantu´,BS1,4, and Hector´ Cruz-Camino, BS1,5 Abstract Newborn screening for the detection of inborn errors of metabolism (IEM), endocrinopathies, hemoglobinopathies, and other disorders is a public health initiative aimed at identifying specific diseases in a timely manner. Mexico initiated newborn screening in 1973, but the national incidence of this group of diseases is unknown or uncertain due to the lack of large sample sizes of expanded newborn screening (ENS) programs and lack of related publications. The incidence of a specific group of IEM, endocrinopathies, hemoglobinopathies, and other disorders in newborns was obtained from a Mexican hospital. These newborns were part of a comprehensive ENS program at Ginequito (a private hospital in Mexico), from January 2012 to August 2014. The retrospective study included the examination of 10 000 newborns’ results obtained from the ENS program (comprising the possible detection of more than 50 screened disorders). The findings were the following: 34 newborns were confirmed with an IEM, endocrinopathies, hemoglobinopathies, or other disorders and 68 were identified as carriers. Consequently, the estimated global incidence for those disorders was 3.4 in 1000 newborns; and the carrier prevalence was 6.8 in 1000. Moreover, a 0.04% false-positive rate was unveiled as soon as diagnostic testing revealed negative results. -

EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies
Focusing on Personalised Medicine EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies Extended carrier screening is an important tool for prospective parents to help them determine their risk of having a child affected with a heritable disease. In many cases, parents aren’t aware they are carriers and have no family history due to the rarity of some diseases in the general population. What is covered by the screening? Genomics For Life offers a comprehensive Extended Carrier Screening test, providing prospective parents with the information they require when planning their pregnancy. Extended Carrier Screening has been shown to detect carriers who would not have been considered candidates for traditional risk- based screening. With a simple mouth swab collection, we are able to test for over 419 genes associated with inherited diseases, including Fragile X Syndrome, Cystic Fibrosis and Spinal Muscular Atrophy. The assay has been developed in conjunction with clinical molecular geneticists, and includes genes listed in the NIH Genetic Test Registry. For a list of genes and disorders covered, please see the reverse of this brochure. If your gene of interest is not covered on our Extended Carrier Screening panel, please contact our friendly team to assist you in finding a gene test panel that suits your needs. Why have Extended Carrier Screening? Extended Carrier Screening prior to pregnancy enables couples to learn about their reproductive risk and consider a complete range of reproductive options, including whether or not to become pregnant, whether to use advanced reproductive technologies, such as preimplantation genetic diagnosis, or to use donor gametes. -

Amino Acid Disorders 105
AMINO ACID DISORDERS 105 Massaro, A. S. (1995). Trypanosomiasis. In Guide to Clinical tions in biological fluids relatively easy. These Neurology (J. P. Mohrand and J. C. Gautier, Eds.), pp. 663– analyzers separate amino acids either by ion-ex- 667. Churchill Livingstone, New York. Nussenzweig, V., Sonntag, R., Biancalana, A., et al. (1953). Ac¸a˜o change chromatography or by high-pressure liquid de corantes tri-fenil-metaˆnicos sobre o Trypanosoma cruzi in chromatography. The results are plotted as a graph vitro: Emprego da violeta de genciana na profilaxia da (Fig. 1). The concentration of each amino acid can transmissa˜o da mole´stia de chagas por transfusa˜o de sangue. then be calculated from the size of the corresponding O Hospital (Rio de Janeiro) 44, 731–744. peak on the graph. Pagano, M. A., Segura, M. J., DiLorenzo, G. A., et al. (1999). Cerebral tumor-like American trypanosomiasis in Most amino acid disorders can be diagnosed by acquired immunodeficiency syndrome. Ann. Neurol. 45, measuring the concentrations of amino acids in 403–406. blood plasma; however, some disorders of amino Rassi, A., Trancesi, J., and Tranchesi, B. (1982). Doenc¸ade acid transport are more easily recognized through the Chagas. In Doenc¸as Infecciosas e Parasita´rias (R. Veroesi, Ed.), analysis of urine amino acids. Therefore, screening 7th ed., pp. 674–712. Guanabara Koogan, Sa˜o Paulo, Brazil. Spina-Franc¸a, A., and Mattosinho-Franc¸a, L. C. (1988). for amino acid disorders is best done using both South American trypanosomiasis (Chagas’ disease). In blood and urine specimens. Occasionally, analysis of Handbook of Clinical Neurology (P. -

Arginine-Provider-Fact-Sheet.Pdf
Arginine (Urea Cycle Disorder) Screening Fact Sheet for Health Care Providers Newborn Screening Program of the Oklahoma State Department of Health What is the differential diagnosis? Argininemia (arginase deficiency, hyperargininemia) What are the characteristics of argininemia? Disorders of arginine metabolism are included in a larger group of disorders, known as urea cycle disorders. Argininemia is an autosomal recessive inborn error of metabolism caused by a defect in the final step in the urea cycle. Most infants are born to parents who are both unknowingly asymptomatic carriers and have NO known history of a urea cycle disorder in their family. The incidence of all urea cycle disorders is estimated to be about 1:8,000 live births. The true incidence of argininemia is not known, but has been estimated between 1:350,000 and 1:1,000,000. Argininemia is usually asymptomatic in the neonatal period, although it can present with mild to moderate hyperammonemia. Untreated, argininemia usually progresses to severe spasticity, loss of ambulation, severe cognitive and intellectual disabilities and seizures Lifelong treatment includes a special diet, and special care during times of illness or stress. What is the screening methodology for argininemia? 1. An amino acid profile by Tandem Mass Spectrometry (MS/MS) is performed on each filter paper. 2. Arginine is the primary analyte. What is an in-range (normal) screen result for arginine? Arginine less than 100 mol/L is NOT consistent with argininemia. See Table 1. TABLE 1. In-range Arginine Newborn Screening Results What is an out-of-range (abnormal) screen for arginine? Arginine > 100 mol/L requires further testing. -

Disease Reference Book
The Counsyl Foresight™ Carrier Screen 180 Kimball Way | South San Francisco, CA 94080 www.counsyl.com | [email protected] | (888) COUNSYL The Counsyl Foresight Carrier Screen - Disease Reference Book 11-beta-hydroxylase-deficient Congenital Adrenal Hyperplasia .................................................................................................................................................................................... 8 21-hydroxylase-deficient Congenital Adrenal Hyperplasia ...........................................................................................................................................................................................10 6-pyruvoyl-tetrahydropterin Synthase Deficiency ..........................................................................................................................................................................................................12 ABCC8-related Hyperinsulinism........................................................................................................................................................................................................................................ 14 Adenosine Deaminase Deficiency .................................................................................................................................................................................................................................... 16 Alpha Thalassemia............................................................................................................................................................................................................................................................. -

TYR I, II, III Act Sheet
Newborn Screening ACT Sheet Increased Tyrosine Tyrosinemia Differential Diagnosis: Tyrosinemia I (hepatorenal); Tyrosinemia II (oculocutaneous); Tyrosinemia III; transient hypertyrosinemia; liver disease. Condition Description: In the hepatorenal form, tyrosine from ingested protein and phenylalanine metabolism cannot be metabolized by fumarylacetoacetate hydrolase to fumaric acid and acetoacetic acid. The resulting fumarylacetoacetate accumulates and is converted to succinylacetone, the diagnostic metabolite, which is liver toxic, and leads to elevated tyrosine. Tyrosinemias II and III are due to other defects in tyrosine degradation. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: plasma quantitative amino acids; urine succinylacetone and liver function tests. • Repeat newborn screen if the second screen has not been done. • Provide family with basic information about tyrosinemia. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino acid analysis will show increased tyrosine in all of the tyrosinemias. Urine organic acid analysis may reveal increased succinylacetone in Tyrosinemia I. Clinical Considerations: Tyrosinemia I is usually asymptomatic in the neonate. If untreated, it will cause liver disease and cirrhosis -
Download This PDF File
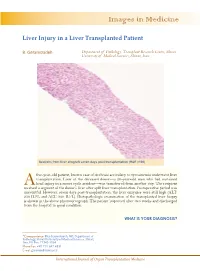
Images in Medicine Liver Injury in a Liver Transplanted Patient B. Geramizadeh Department of Pathology, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Sections from liver allograft seven days post-transplantation (H&E ×100) five-year-old patient, known case of cirrhosis secondary to tyrosinemia underwent liver transplantation. Liver of the deceased donor—a 20-year-old man who had sustained A head injury in a motor cycle accident—was transferred from another city. The recipient received a segment of the donor’s liver after split liver transplantation. Postoperative period was uneventful. However, seven days post-transplantation, the liver enzymes were still high (ALT: 250 IU/L and ALT: 320 IU/L). Histopathologic examination of the transplanted liver biopsy is shown in the above photomicrograph. The patient improved after two weeks and discharged from the hospital in good condition. WHAT IS YOUR DIAGNOSIS? *Correspondence: Bita Geramizadeh, MD, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran. PO Box: 71345-1864 Phone/Fax: +98-711-647-4331 E-mail: [email protected] International Journal of Organ Transplantation Medicine B. Geramizadeh DIAGNOSIS: PRESERVATION/REPERFUSION INJURY dvances in organ preservation have duct obstruction. The distinctive pattern of reduced preservation injury. Neverthe- bile ductular cholestasis is that it is usually as- Aless, when storage time exceeds 10 to sociated with sepsis. Drug toxicity can mimic 12 hours, post-transplantation complications, every change in the liver and should always be due to preservation/reperfusion injury, be- excluded [5]. come more common [1]. In our patient, prolonged cold ischemic time Ischemic injury to the graft is divided into (transfer of the liver from another city) and cold ischemia—secondary to prolonged pres- probably small for size graft (split liver trans- ervation—and warm ischemia, which occurs plantation) were predisposing factors. -

What Disorders Are Screened for by the Newborn Screen?
What disorders are screened for by the newborn screen? Endocrine Disorders The endocrine system is important to regulate the hormones in our bodies. Hormones are special signals sent to various parts of the body. They control many things such as growth and development. The goal of newborn screening is to identify these babies early so that treatment can be started to keep them healthy. To learn more about these specific disorders please click on the name of the disorder below: English: Congenital Adrenal Hyperplasia Esapnol Hiperplasia Suprarrenal Congenital - - http://www.newbornscreening.info/Parents/otherdisorders/CAH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CAH.html - Congenital Hypothyroidism (Hipotiroidismo Congénito) - http://www.newbornscreening.info/Parents/otherdisorders/CH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CH.html Hematologic Conditions Hemoglobin is a special part of our red blood cells. It is important for carrying oxygen to the parts of the body where it is needed. When people have problems with their hemoglobin they can have intense pain, and they often get sick more than other children. Over time, the lack of oxygen to the body can cause damage to the organs. The goal of newborn screening is to identify babies with these conditions so that they can get early treatment to help keep them healthy. To learn more about these specific disorders click here (XXX). - Sickle Cell Anemia (Anemia de Célula Falciforme) - http://www.newbornscreening.info/Parents/otherdisorders/SCD.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/SCD.html - SC Disease (See Previous Link) - Sickle Beta Thalassemia (See Previous Link) Enzyme Deficiencies Enzymes are special proteins in our body that allow for chemical reactions to take place. -

Inborn Errors of Metabolism
Inborn Errors of Metabolism Mary Swift, Registered Dietician (R.D.) -------------------------------------------------------------------------------- Definition Inborn Errors of Metabolism are defects in the mechanisms of the body which break down specific parts of food into chemicals the body is able to use. Resulting in the buildup of toxins in the body. Introduction Inborn Errors of Metabolism (IEM) are present at birth and persist throughout life. They result from a failure in the chemical changes that are metabolism. They often occur in members of the same family. Parents of affected individuals are often related. The genes that cause IEM are autosomal recessive. Thousands of molecules in each cell of the body are capable of reactions with other molecules in the cell. Special proteins called enzymes speed up these reactions. Each enzyme speeds up the rate of a specific type of reaction. A single gene made up of DNA controls the production of each enzyme. Specific arrangements of the DNA correspond to specific amino acids. This genetic code determines the order in which amino acids are put together to form proteins in the body. A change in the arrangement of DNA within the gene can result in a protein or enzyme that is not able to carry out its function. The result is a change in the ability of the cell to complete a particular reaction resulting in a metabolic block. The areas of the cell these errors occur determine the severity of the consequences of the break down in metabolism. For example if the error occurs in critical areas of energy production, the cell will die.