Newborn Screening Laboratory Manual of Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperammonemia in Review: Pathophysiology, Diagnosis, and Treatment
Pediatr Nephrol DOI 10.1007/s00467-011-1838-5 EDUCATIONAL REVIEW Hyperammonemia in review: pathophysiology, diagnosis, and treatment Ari Auron & Patrick D. Brophy Received: 23 September 2010 /Revised: 9 January 2011 /Accepted: 12 January 2011 # IPNA 2011 Abstract Ammonia is an important source of nitrogen and is the breakdown and catabolism of dietary and bodily proteins, required for amino acid synthesis. It is also necessary for respectively. In healthy individuals, amino acids that are not normal acid-base balance. When present in high concentra- needed for protein synthesis are metabolized in various tions, ammonia is toxic. Endogenous ammonia intoxication chemical pathways, with the rest of the nitrogen waste being can occur when there is impaired capacity of the body to converted to urea. Ammonia is important for normal animal excrete nitrogenous waste, as seen with congenital enzymatic acid-base balance. During exercise, ammonia is produced in deficiencies. A variety of environmental causes and medica- skeletal muscle from deamination of adenosine monophos- tions may also lead to ammonia toxicity. Hyperammonemia phate and amino acid catabolism. In the brain, the latter refers to a clinical condition associated with elevated processes plus the activity of glutamate dehydrogenase ammonia levels manifested by a variety of symptoms and mediate ammonia production. After formation of ammonium signs, including significant central nervous system (CNS) from glutamine, α-ketoglutarate, a byproduct, may be abnormalities. Appropriate and timely management requires a degraded to produce two molecules of bicarbonate, which solid understanding of the fundamental pathophysiology, are then available to buffer acids produced by dietary sources. differential diagnosis, and treatment approaches available. -

Disorders Included in the Newborn Screening Panel Disorders
Disorders Included in the Newborn Screening Panel Disorders Detected by Tandem Mass Spectrometry Acylcarnitine Profile Amino Acid Profile Fatty Acid Oxidation Disorders Amino Acid Disorders Carnitine/Acylcarnitine Translocase Deficiency Argininemia 1 Carnitine Palmitoyl Transferase Deficiency Type I Argininosuccinic Aciduria 1 3-Hydroxy Long Chain Acyl-CoA Dehydrogenase 5-Oxoprolinuria 1 Deficiency Carbamoylphosphate Synthetase Deficiency 1 2,4-Dienoyl-CoA Reductase Deficiency Citrullinemia Medium Chain Acyl-CoA Dehydrogenase Deficiency Homocystinuria Multiple Acyl-CoA Dehydrogenase Deficiency Hypermethioninemia Neonatal Carnitine Palmitoyl Transferase Deficiency Hyperammonemia, Hyperornithinemia, Homocitrullinuria Type II Syndrome1 1 Short Chain Acyl-CoA Dehydrogenase Deficiency Hyperornithinemia with Gyral Atrophy Short Chain Hydroxy Acyl-CoA Dehydrogenase Maple Syrup Urine Disease Deficiency Phenylketonuria Trifunctional Protein Deficiency Classical/Hyperphenylalaninemia Very Long Chain Acyl-CoA Dehydrogenase Deficiency Biopterin Cofactor Deficiencies Tyrosinemia Organic Acid Disorders Transient Neonatal Tyrosinemia 2 Tyrosinemia Type I 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency Tyrosinemia Type II Glutaric Acidemia Type I Tyrosinemia Type III Isobutyryl-CoA Dehydrogenase Deficiency Isovaleric Acidemia 2-Methylbutyryl-CoA Dehydrogenase Deficiency 3-Methylcrotonyl-CoA Carboxylase Deficiency Other Observations 3-Methylglutaconyl-CoA Hydratase Deficiency Methylmalonic Acidemias Hyperalimentation Methylmalonyl-CoA Mutase Deficiency -

Glutaric Acidemia Type 1
Glutaric acidemia type 1 What is glutaric acidemia type 1? Glutaric acidemia type 1 is an inherited disease characterized by episodes of severe brain dysfunction that result in spasticity, low muscle tone, and seizures.1,2 Individuals with glutaric acidemia type 1 have defects in the glutaryl-CoA dehydrogenase enzyme, which breaks down the amino acids lysine, hydroxylysine, and tryptophan. The symptoms of glutaric acidemia type 1 are due to the build-up of these amino acids and their metabolites in the body, primarily affecting the brain.1 Glutaric acidemia type 1 is also known as glutaric aciduria type 1.2 What are the symptoms of glutaric acidemia type 1 and what treatment is available? The severity of symptoms of glutaric acidemia type 1 can vary widely, even within families. Newborns may have macrocephaly (large head size) with no other signs or symptoms. Symptoms typically begin within months after birth and are often triggered by illness or fasting. Symptoms may include2: • Hypotonia (low muscle tone) • Feeding difficulties • Poor growth • Swelling of the brain • Spasticity (abnormally tight muscles) • Dystonia (sustained muscle contractions causing twisting movements and abnormal posture) • Seizures • Developmental delays • Coma, and possibly death, especially if untreated Individuals tend to have a reduced life expectancy. Approximately 10% of individuals die within the first decade; more than half do not survive past 35 years of age. 3 There is no cure for glutaric acidemia type 1, and treatment is aimed at preventing episodes of brain dysfunction and seizures. Treatment generally includes a low protein diet and nutrition supplements, and a feeding tube may be required for some individuals. -

Arginine-Provider-Fact-Sheet.Pdf
Arginine (Urea Cycle Disorder) Screening Fact Sheet for Health Care Providers Newborn Screening Program of the Oklahoma State Department of Health What is the differential diagnosis? Argininemia (arginase deficiency, hyperargininemia) What are the characteristics of argininemia? Disorders of arginine metabolism are included in a larger group of disorders, known as urea cycle disorders. Argininemia is an autosomal recessive inborn error of metabolism caused by a defect in the final step in the urea cycle. Most infants are born to parents who are both unknowingly asymptomatic carriers and have NO known history of a urea cycle disorder in their family. The incidence of all urea cycle disorders is estimated to be about 1:8,000 live births. The true incidence of argininemia is not known, but has been estimated between 1:350,000 and 1:1,000,000. Argininemia is usually asymptomatic in the neonatal period, although it can present with mild to moderate hyperammonemia. Untreated, argininemia usually progresses to severe spasticity, loss of ambulation, severe cognitive and intellectual disabilities and seizures Lifelong treatment includes a special diet, and special care during times of illness or stress. What is the screening methodology for argininemia? 1. An amino acid profile by Tandem Mass Spectrometry (MS/MS) is performed on each filter paper. 2. Arginine is the primary analyte. What is an in-range (normal) screen result for arginine? Arginine less than 100 mol/L is NOT consistent with argininemia. See Table 1. TABLE 1. In-range Arginine Newborn Screening Results What is an out-of-range (abnormal) screen for arginine? Arginine > 100 mol/L requires further testing. -

TYR I, II, III Act Sheet
Newborn Screening ACT Sheet Increased Tyrosine Tyrosinemia Differential Diagnosis: Tyrosinemia I (hepatorenal); Tyrosinemia II (oculocutaneous); Tyrosinemia III; transient hypertyrosinemia; liver disease. Condition Description: In the hepatorenal form, tyrosine from ingested protein and phenylalanine metabolism cannot be metabolized by fumarylacetoacetate hydrolase to fumaric acid and acetoacetic acid. The resulting fumarylacetoacetate accumulates and is converted to succinylacetone, the diagnostic metabolite, which is liver toxic, and leads to elevated tyrosine. Tyrosinemias II and III are due to other defects in tyrosine degradation. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: plasma quantitative amino acids; urine succinylacetone and liver function tests. • Repeat newborn screen if the second screen has not been done. • Provide family with basic information about tyrosinemia. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino acid analysis will show increased tyrosine in all of the tyrosinemias. Urine organic acid analysis may reveal increased succinylacetone in Tyrosinemia I. Clinical Considerations: Tyrosinemia I is usually asymptomatic in the neonate. If untreated, it will cause liver disease and cirrhosis -
Download This PDF File
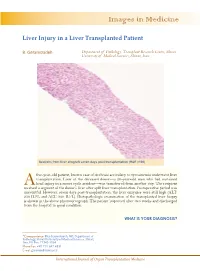
Images in Medicine Liver Injury in a Liver Transplanted Patient B. Geramizadeh Department of Pathology, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Sections from liver allograft seven days post-transplantation (H&E ×100) five-year-old patient, known case of cirrhosis secondary to tyrosinemia underwent liver transplantation. Liver of the deceased donor—a 20-year-old man who had sustained A head injury in a motor cycle accident—was transferred from another city. The recipient received a segment of the donor’s liver after split liver transplantation. Postoperative period was uneventful. However, seven days post-transplantation, the liver enzymes were still high (ALT: 250 IU/L and ALT: 320 IU/L). Histopathologic examination of the transplanted liver biopsy is shown in the above photomicrograph. The patient improved after two weeks and discharged from the hospital in good condition. WHAT IS YOUR DIAGNOSIS? *Correspondence: Bita Geramizadeh, MD, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran. PO Box: 71345-1864 Phone/Fax: +98-711-647-4331 E-mail: [email protected] International Journal of Organ Transplantation Medicine B. Geramizadeh DIAGNOSIS: PRESERVATION/REPERFUSION INJURY dvances in organ preservation have duct obstruction. The distinctive pattern of reduced preservation injury. Neverthe- bile ductular cholestasis is that it is usually as- Aless, when storage time exceeds 10 to sociated with sepsis. Drug toxicity can mimic 12 hours, post-transplantation complications, every change in the liver and should always be due to preservation/reperfusion injury, be- excluded [5]. come more common [1]. In our patient, prolonged cold ischemic time Ischemic injury to the graft is divided into (transfer of the liver from another city) and cold ischemia—secondary to prolonged pres- probably small for size graft (split liver trans- ervation—and warm ischemia, which occurs plantation) were predisposing factors. -

What Disorders Are Screened for by the Newborn Screen?
What disorders are screened for by the newborn screen? Endocrine Disorders The endocrine system is important to regulate the hormones in our bodies. Hormones are special signals sent to various parts of the body. They control many things such as growth and development. The goal of newborn screening is to identify these babies early so that treatment can be started to keep them healthy. To learn more about these specific disorders please click on the name of the disorder below: English: Congenital Adrenal Hyperplasia Esapnol Hiperplasia Suprarrenal Congenital - - http://www.newbornscreening.info/Parents/otherdisorders/CAH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CAH.html - Congenital Hypothyroidism (Hipotiroidismo Congénito) - http://www.newbornscreening.info/Parents/otherdisorders/CH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CH.html Hematologic Conditions Hemoglobin is a special part of our red blood cells. It is important for carrying oxygen to the parts of the body where it is needed. When people have problems with their hemoglobin they can have intense pain, and they often get sick more than other children. Over time, the lack of oxygen to the body can cause damage to the organs. The goal of newborn screening is to identify babies with these conditions so that they can get early treatment to help keep them healthy. To learn more about these specific disorders click here (XXX). - Sickle Cell Anemia (Anemia de Célula Falciforme) - http://www.newbornscreening.info/Parents/otherdisorders/SCD.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/SCD.html - SC Disease (See Previous Link) - Sickle Beta Thalassemia (See Previous Link) Enzyme Deficiencies Enzymes are special proteins in our body that allow for chemical reactions to take place. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Glutaric Acidemia Type 1: Treatment and Outcome of 168 Patients Over Three Decades
University of Massachusetts Medical School eScholarship@UMMS Open Access Publications by UMMS Authors 2020-11-01 Glutaric acidemia type 1: Treatment and outcome of 168 patients over three decades Kevin A. Strauss University of Massachusetts Medical School Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Congenital, Hereditary, and Neonatal Diseases and Abnormalities Commons, Nutritional and Metabolic Diseases Commons, and the Pediatrics Commons Repository Citation Strauss KA. (2020). Glutaric acidemia type 1: Treatment and outcome of 168 patients over three decades. Open Access Publications by UMMS Authors. https://doi.org/10.1016/j.ymgme.2020.09.007. Retrieved from https://escholarship.umassmed.edu/oapubs/4429 Creative Commons License This work is licensed under a Creative Commons Attribution 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Publications by UMMS Authors by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Molecular Genetics and Metabolism 131 (2020) 325–340 Contents lists available at ScienceDirect Molecular Genetics and Metabolism journal homepage: www.elsevier.com/locate/ymgme Glutaric acidemia type 1: Treatment and outcome of 168 patients over three T decades ⁎ Kevin A. Straussa,b,c, , Katie B. Williamsa, Vincent J. Carsona,b, Laura Poskitta,b, Lauren E. Bowsera, Millie Younga, Donna L. Robinsona, -

AMERICAN ACADEMY of PEDIATRICS Reimbursement For
AMERICAN ACADEMY OF PEDIATRICS POLICY STATEMENT Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health of All Children Committee on Nutrition Reimbursement for Foods for Special Dietary Use ABSTRACT. Foods for special dietary use are recom- DEFINITION OF FOODS FOR SPECIAL mended by physicians for chronic diseases or conditions DIETARY USE of childhood, including inherited metabolic diseases. Al- The US Food and Drug Administration, in the though many states have created legislation requiring Code of Federal Regulations,2 defines special dietary reimbursement for foods for special dietary use, legisla- use of foods as the following: tion is now needed to mandate consistent coverage and reimbursement for foods for special dietary use and re- a. Uses for supplying particular dietary needs that lated support services with accepted medical benefit for exist by reason of a physical, physiologic, patho- children with designated medical conditions. logic, or other condition, including but not limited to the conditions of diseases, convalescence, preg- ABBREVIATION. AAP, American Academy of Pediatrics. nancy, lactation, allergic hypersensitivity to food, [and being] underweight and overweight; b. Uses for supplying particular dietary needs which BACKGROUND exist by reason of age, including but not limited to pecial foods are recommended by physicians to the ages of infancy and childhood; foster normal growth and development in some c. Uses for supplementing or fortifying the ordinary or usual diet with any vitamin, mineral, or other children and to prevent serious disability and S dietary property. Any such particular use of a even death in others. Many of these special foods are food is a special dietary use, regardless of whether technically specialized formulas for which there may such food also purports to be or is represented for be a relatively small market, which makes them more general use. -

Defects in Amino Acid Catabolism and the Urea Cycle
Handbook of Clinical Neurology, Vol. 113 (3rd series) Pediatric Neurology Part III O. Dulac, M. Lassonde, and H.B. Sarnat, Editors © 2013 Elsevier B.V. All rights reserved Chapter 181 Defects in amino acid catabolism and the urea cycle GEORG F. HOFFMANN* AND STEFAN KO¨ LKER Department of General Pediatrics, University Children’s Hospital Heidelberg, Heidelberg, Germany INTRODUCTION organizations can offer support. Since treatment is time- and cost-intensive, often lifelong, and mostly Defects in amino acid catabolism and the urea cycle performed at home, regular training and support of result in an accumulation of metabolites upstream of patients and their families is essential. Unfortunately, as the defective enzyme (amino acids (AAs), organic acids for all chronic diseases, average compliance with recom- and/or ammonia) causing intoxication. Phenylketonuria mendations has been found as low as 50%, e.g., in PKU. (PKU) is the most frequent AA disorder (AAD) in Individual defects in AA catabolism and the urea Caucasians. Breakdown of AAs results in the release cycle usually have a low prevalence except for some of ammonia that is detoxified by the urea cycle. Hyper- communities with high consanguinity rates. However, ammonemia is the biochemical hallmark of urea cycle the cumulative prevalence of these disorders is consider- defects (UCDs). able (i.e., at least> 1:2000 newborns; Schulze et al., After an uneventful pregnancy and an initially asymp- 2003). Detailed information on diagnosis, genetic test- tomatic period, the onset of symptoms in these disorders ing, treatment and follow-up is available at the following varies from neonatal metabolic decompensation to late online databases: onset in adulthood. -

Evidence Table of Systematic Literature Search* According to SIGN Level
027/018 – Glutarazidurie Typ I aktueller Stand: 06/2016 Evidence table of systematic literature search* according to SIGN Level Listed are published data since January 1st, 2011. For the time period from January 1st 1975 – December 31th 2010, please see the evidence table of systematic literature research generated for the 1st guideline revision in 2011 (page 36). Level 2++, 1++, 1+, 1-. None Level 2+. Evidence from well-conducted case-control or cohort studies with a low risk of confounding, bias, or chance and a moderate probability that the relationship is causal. First Title Study Design Patients (n) Clinical End Results / Conclusions Bias Confounding SIGN Author points (according to (according to Level GRADE) GRADE) Boy et al. A cross-sectional Cross sectional 30 patients Dystonia was BADS scores correlated with Effect of severe 2+ (2015) controlled study diagnosed by categorized using speed tests but not with tests dystonia might be Orphanet developmental study newborn screening the Barry-Albright- measuring stability or higher underestimated; J Rare Dis; of neuropsychological (n = 13), high-risk Dystonia Scale (BADS) cognitive functions 10:163 functions in patients screening (n = 3) or simple reaction time Developmental functions of False negative with glutaric aciduria targeted metabolic (SRT), continuous patients differed from findings are type I testing (n = 14) performance (CP), controls for SRT and VS but possible due to visual working not for VWM. Dystonic unequal sizes of n=13 dystonic memory patients were slower in SRT age groups patients, n=17 (VWM), visual-motor and CP but reached their asymptomatic coordination asymptote of performance patients (Tracking) and visual similar to asymptomatic search (VS).