Orfadin, Nitisinone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxicidade De Herbicidas Pós- Emergentes Em Cultivares De Feijão-Caupi
TOXICIDADE DE HERBICIDAS PÓS- EMERGENTES EM CULTIVARES DE FEIJÃO-CAUPI THIAGO REIS PRADO 2016 THIAGO REIS PRADO TOXICIDADE DE HERBICIDAS PÓS-EMERGENTES EM CULTIVARES DE FEIJÃO-CAUPI Dissertação apresentada à Universidade Estadual do Sudoeste da Bahia, Campus de Vitória da Conquista, para obtenção do título de Mestre em Agronomia. Orientador: Prof. D.Sc.Alcebíades Rebouças São José VITÓRIA DA CONQUISTA BAHIA - BRASIL 2016 P915t Prado, Thiago Reis. Toxicidade de herbicidas pós-emergentes em cultivares de feijão-caupi. / Thiago Reis Prado. 55f. Orientador (a): D.Sc. Alcebíades Rebouças São José. Dissertação (mestrado) – Universidade Estadual do Sudoeste da Bahia, Programa de Pós-graduação em Agronomia, área de concentração em Fitotecnia. Vitória da Conquista, 2016. Referências: f. 50-55. 1. Vigna unguiculata - Cultivo. 2. Controle químico. 3. Planta daninha. 4. Fitotoxidade. I. São José, Alcebíades Rebouças II. Universidade Estadual do Sudoeste da Bahia, Programa de Pós-Graduação em Agronomia, área de Concentração em Fitotecnia. III. T. CDD: 633.33 Catalogação na fonte : Juliana Teixeira de Assunção – CRB 5/18 90 UESB – Campus Vitória da Conquista - BA Aos meus pais, Paulo Soares Prado e Maria José Reis Prado, que, sempre dedicados à minha educação e fortes na fé, atuam de forma singular com seus ensinamentos e suas orações. Dedico Agradecimentos A Deus, por iluminar minha vida e guiar por todos os caminhos. Aos meus pais, Paulo Soares Prado e Maria José Reis Prado, pelo amor e por apoiarem sempre as decisões para realização dos meus sonhos. Ao Prof. Dr. Alcebíades Rebouças São José, pela confiança, orientação, compreensão e amizade durante todo o curso do mestrado. -

Newborn Screening Laboratory Manual of Services
Newborn Screening Laboratory Manual of Services Test Panel: Please see the following links for a detailed description of testing in the Newborn Screening section. Information about the Newborn Screening program is available here. Endocrine Disorders Congenital adrenal hyperplasia (CAH) Congenital hypothyroidism (TSH) Hemoglobinopathies Sickle cell disease (FS) Alpha (Barts) Sickle βeta Thalassemia (FSA) Other sickling hemoglobinopathies such as: FAS FAC FAD FAE Homozygous conditions such as: FC FD FE Metabolic Disorders Biotinidase deficiency Galactosemia Cystic fibrosis (CF) first tier screening for elevated immunoreactive trypsinogen (IRT) Cystic fibrosis second tier genetic mutation analysis on the top 4% IRT concentrations. Current alleles detected : F508del, I507del, G542X, G85E, R117H, 621+1G->T, 711+1G->T, R334W, R347P, A455E, 1717-1G->A, R560T, R553X, G551D, 1898+1G->A, 2184delA, 2789+5G->A, 3120+1G->A, R1162X, 3659delC, 3849+10kbC->T, W1282X, N1303K, IVS polyT T5/T7/T9 *Currently validating a mutation panel that includes the above alleles in addition to the following: 1078delT, Y122X, 394delTT, R347H, M1101K, S1255X, 1898+5G->T, 2183AA->G, 2307insA, Y1092X, 3876delA, 3905insT, S549N, S549R_1645A->C, S549R-1647T->G, S549R-1647T->G, V520F, A559T, 1677delTA, 2055del9->A, 2143delT, 3199del6, 406-1G->A, 935delA, D1152H, CFTRdele2, E60X, G178R, G330X, K710X, L206W, Q493X, Q890X, R1066C, R1158X, R75X, S1196X, W1089X, G1244E, G1349D, G551S, R560KT, S1251N, S1255P Amino acid disorders Phenylketonuria (PKU) / Hyperphenylalaninemia Maple -

TYR I, II, III Act Sheet
Newborn Screening ACT Sheet Increased Tyrosine Tyrosinemia Differential Diagnosis: Tyrosinemia I (hepatorenal); Tyrosinemia II (oculocutaneous); Tyrosinemia III; transient hypertyrosinemia; liver disease. Condition Description: In the hepatorenal form, tyrosine from ingested protein and phenylalanine metabolism cannot be metabolized by fumarylacetoacetate hydrolase to fumaric acid and acetoacetic acid. The resulting fumarylacetoacetate accumulates and is converted to succinylacetone, the diagnostic metabolite, which is liver toxic, and leads to elevated tyrosine. Tyrosinemias II and III are due to other defects in tyrosine degradation. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: plasma quantitative amino acids; urine succinylacetone and liver function tests. • Repeat newborn screen if the second screen has not been done. • Provide family with basic information about tyrosinemia. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino acid analysis will show increased tyrosine in all of the tyrosinemias. Urine organic acid analysis may reveal increased succinylacetone in Tyrosinemia I. Clinical Considerations: Tyrosinemia I is usually asymptomatic in the neonate. If untreated, it will cause liver disease and cirrhosis -
Download This PDF File
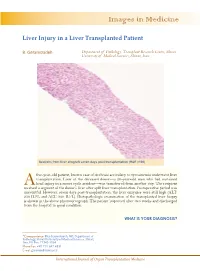
Images in Medicine Liver Injury in a Liver Transplanted Patient B. Geramizadeh Department of Pathology, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Sections from liver allograft seven days post-transplantation (H&E ×100) five-year-old patient, known case of cirrhosis secondary to tyrosinemia underwent liver transplantation. Liver of the deceased donor—a 20-year-old man who had sustained A head injury in a motor cycle accident—was transferred from another city. The recipient received a segment of the donor’s liver after split liver transplantation. Postoperative period was uneventful. However, seven days post-transplantation, the liver enzymes were still high (ALT: 250 IU/L and ALT: 320 IU/L). Histopathologic examination of the transplanted liver biopsy is shown in the above photomicrograph. The patient improved after two weeks and discharged from the hospital in good condition. WHAT IS YOUR DIAGNOSIS? *Correspondence: Bita Geramizadeh, MD, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran. PO Box: 71345-1864 Phone/Fax: +98-711-647-4331 E-mail: [email protected] International Journal of Organ Transplantation Medicine B. Geramizadeh DIAGNOSIS: PRESERVATION/REPERFUSION INJURY dvances in organ preservation have duct obstruction. The distinctive pattern of reduced preservation injury. Neverthe- bile ductular cholestasis is that it is usually as- Aless, when storage time exceeds 10 to sociated with sepsis. Drug toxicity can mimic 12 hours, post-transplantation complications, every change in the liver and should always be due to preservation/reperfusion injury, be- excluded [5]. come more common [1]. In our patient, prolonged cold ischemic time Ischemic injury to the graft is divided into (transfer of the liver from another city) and cold ischemia—secondary to prolonged pres- probably small for size graft (split liver trans- ervation—and warm ischemia, which occurs plantation) were predisposing factors. -

What Disorders Are Screened for by the Newborn Screen?
What disorders are screened for by the newborn screen? Endocrine Disorders The endocrine system is important to regulate the hormones in our bodies. Hormones are special signals sent to various parts of the body. They control many things such as growth and development. The goal of newborn screening is to identify these babies early so that treatment can be started to keep them healthy. To learn more about these specific disorders please click on the name of the disorder below: English: Congenital Adrenal Hyperplasia Esapnol Hiperplasia Suprarrenal Congenital - - http://www.newbornscreening.info/Parents/otherdisorders/CAH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CAH.html - Congenital Hypothyroidism (Hipotiroidismo Congénito) - http://www.newbornscreening.info/Parents/otherdisorders/CH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CH.html Hematologic Conditions Hemoglobin is a special part of our red blood cells. It is important for carrying oxygen to the parts of the body where it is needed. When people have problems with their hemoglobin they can have intense pain, and they often get sick more than other children. Over time, the lack of oxygen to the body can cause damage to the organs. The goal of newborn screening is to identify babies with these conditions so that they can get early treatment to help keep them healthy. To learn more about these specific disorders click here (XXX). - Sickle Cell Anemia (Anemia de Célula Falciforme) - http://www.newbornscreening.info/Parents/otherdisorders/SCD.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/SCD.html - SC Disease (See Previous Link) - Sickle Beta Thalassemia (See Previous Link) Enzyme Deficiencies Enzymes are special proteins in our body that allow for chemical reactions to take place. -

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -

Orfadin, INN-Nitisinone
SCIENTIFIC DISCUSSION 1. Introduction 1.1 Problem statement Hereditary tyrosinaemia type 1 (HT-1) is a devastating inherited disease, mainly of childhood. It is characterised by severe liver dysfunction, impaired coagulation, painful neurological crises, renal tubular dysfunction and a considerable risk of hepatocellular carcinoma (Weinberg et al. 1976, Halvorsen 1990, Kvittingen 1991, van Spronsen et al. 1994, Mitchell et al. 1995). The condition is caused by an inborn error in the final step of the tyrosine degradation pathway (Lindblad et al. 1977). The incidence of HT-1 in Europe and North America is about one in 100,000 births, although in certain areas the incidence is considerably higher. In the province of Quebec, Canada, it is about one in 20,000 births (Mitchell et al. 1995). The mode of inheritance is autosomal recessive. The primary enzymatic defect in HT-1 is a reduced activity of fumarylacetoacetate hydrolase (FAH) in the liver, the last enzyme in the tyrosine degradation pathway. As a consequence, fumaylacetoacetate (FAA) and maleylacetoacetate (MAA), upstream of the enzymatic block, accumulate. Both intermediates are highly reactive and unstable and cannot be detected in the serum or urine of affected children. Degradation products of MAA and FAA are succinylacetone (SA) and succinylacetoacetate (SAA) which are (especially SA) toxic, and which are measurable in the serum and urine and are hallmarks of the disease. SA is also an inhibitor of Porphobilinogen synthase (PBG), leading to an accumulation of 5-aminolevulinate (5-ALA) which is thought to be responsible for the neurologic crises resembling the crises of the porphyrias. The accumulation of toxic metabolites starts at birth and the severity of phenotype is reflected in the age of onset of symptoms (Halvorsen 1990, van Spronsen et al. -

Quantification of the Flux of Tyrosine Pathway Metabolites During Nitisinone Treatment of Alkaptonuria
www.nature.com/scientificreports OPEN Quantifcation of the fux of tyrosine pathway metabolites during nitisinone treatment of Received: 21 August 2018 Accepted: 20 June 2019 Alkaptonuria Published: xx xx xxxx A. M. Milan 1,2, A. T. Hughes1,2, A. S. Davison 1,2, M. Khedr 1, J. Rovensky3, E. E. Psarelli4, T. F. Cox4, N. P. Rhodes2, J. A. Gallagher2 & L. R. Ranganath1,2 Nitisinone decreases homogentisic acid (HGA) in Alkaptonuria (AKU) by inhibiting the tyrosine metabolic pathway in humans. The efect of diferent daily doses of nitisinone on circulating and 24 h urinary excretion of phenylalanine (PA), tyrosine (TYR), hydroxyphenylpyruvate (HPPA), hydroxyphenyllactate (HPLA) and HGA in patients with AKU was studied over a four week period. Forty AKU patients, randomised into fve groups of eight patients, received doses of 1, 2, 4 or 8 mg of nitisinone daily, or no drug (control). Metabolites were analysed by tandem mass spectrometry in 24 h urine and serum samples collected before and after nitisinone. Serum metabolites were corrected for total body water and the sum of 24 hr urine plus total body water metabolites of PA, TYR, HPPA, HPLA and HGA were determined. Body weight and urine urea were used to check on stability of diet and metabolism over the 4 weeks of study. The sum of quantities of urine metabolites (PA, TYR, HPPA, HPLA and HGA) were similar pre- and post-nitisinone. The sum of total body water metabolites were signifcantly higher post-nitisinone (p < 0.0001) at all doses. Similarly, combined 24 hr urine:total body water ratios for all analytes were signifcantly higher post-nitisinone, compared with pre-nitisinone baseline for all doses (p = 0.0002 – p < 0.0001). -

Nityr, Orfadin) Reference Number: CP.PHAR.132 Effective Date: 08.28.18 Last Review Date: 11.20 Line of Business: Commercial, HIM, Medicaid Revision Log
Clinical Policy: Nitisinone (Nityr, Orfadin) Reference Number: CP.PHAR.132 Effective Date: 08.28.18 Last Review Date: 11.20 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Nitisinone (Nityr™, Orfadin®) is a hydroxy-phenylpyruvate dioxygenase inhibitor. FDA Approved Indication(s) Nityr and Orfadin are indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 (HT-1) in combination with dietary restriction of tyrosine and phenylalanine. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Nityr and Orfadin are medically necessary when the following criteria are met: I. Initial Approval Criteria A. Hereditary Tyrosinemia Type 1 (must meet all): 1. Diagnosis of HT-1; 2. Prescribed by or in consultation with an endocrinologist or a metabolic or genetic disease specialist; 3. Request is for use as an adjunct to dietary restriction of tyrosine and phenylalanine; 4. Dose does not exceed 2 mg/kg per day. Approval duration: 6 months B. Other diagnoses/indications 1. Refer to the off-label use policy for the relevant line of business if diagnosis is NOT specifically listed under section III (Diagnoses/Indications for which coverage is NOT authorized): CP.CPA.09 for commercial, HIM.PHAR.21 for health insurance marketplace, and CP.PMN.53 for Medicaid. II. Continued Therapy A. Hereditary Tyrosinemia Type 1 (must meet all): 1. -

Effect of Post-Emergence Application of Dichlorophenoxy Acetic Acid (2,4-D) Herbicide on Growth and Development of Three Weeds Associated with Maize Plant Growth
520 Current Science International 3(4): 520-525, 2014 ISSN: 2077-4435 Effect of Post-emergence Application of Dichlorophenoxy acetic acid (2,4-D) Herbicide on Growth and Development of three Weeds Associated with Maize plant growth Hanan M. Abou El-ghit Botany and Microbiology department, Science Faculty, Helwan University, Cairo, Egypt. ABSTRACT Post emergence application of 2,4-Dichlorophenoxyacetic acid (2,4-D) as a herbicide even at low concentrations showed an effective control on three weeds (slender amaranth, goosefoot and prickly burweed) associated with maize growth. 2,4-D was applied at 0.0, 125, 250, 500 and 1000 ppm. 250 ppm 2,4-D treatment significantly decreased numbers and fresh weights of the three weeds by 50% approximately. At 1000 ppm 2,4- D, there were sever reductions in weeds numbers, fresh weights and photosynthetic pigments contents of the three weeds. Maize was undamaged by any tested 2,4-D treatment. Fresh weights and photosynthetic pigments contents of maize were favored significantly after application of 2,4-D on the associated weeds at all tested rates. Key words: Maize, Weeds, Chemical control, Herbicides, Phenoxy herbicides, 2,4-Dichlorophenoxy acetic acid (2,4- D), Auxins. Introduction Maize (Zea mays L.) is the second most important cereal crop in Egypt in terms of total food production. It is grown for fodder as well as for grain. The grains of maize are used in a variety of ways by the human beings. Weeds are a major problem in crop production and drastically decrease crop yield. Therefore, weed control is an important management practice for maize production that should be carried out to ensure optimum grain yield. -

Tyrosinemia (Type I) – Amino Acid Disorder
Tyrosinemia (Type I) – Amino Acid Disorder What are amino acid disorders? Kidney disease may lead to rickets, a bone The amino acid disorders are a class of disease. The nerves may also be affected. inherited metabolic conditions that occur when Some babies may have a rapid heart rate, certain amino acids either cannot be broken breathing difficulties, and seizures. down or cannot be produced by the body, Occasionally, individuals with liver damage resulting in the toxic accumulation of some have a higher risk of developing liver cancer. substances and the deficiency of other Acute liver and kidney damage can lead to substances. death. What is tyrosinemia? How is the diagnosis confirmed? In tyrosinemia, the amino acid tyrosine cannot The diagnosis is confirmed by measuring the be broken down properly, leading to a toxic levels of amino acids in the blood and organic accumulation of this amino acid and its acids in the urine. The finding of metabolites in the body. succinylacetone in the urine is diagnostic. Enzyme testing and genetic testing of the FAH What is its incidence? gene may also be used to confirm the Tyrosinemia affects about 1 out every 100,000 diagnosis. Diagnostic testing is arranged by babies born in BC. Although tyrosinemia specialists at BC Children’s Hospital. occurs in all ethnic groups, it is more common in certain populations. Its incidence has been What is the treatment of the disease? reported as high as 1 in 2,000 in the French Children with tyrosinemia are treated with a Canadian population living in the Saguenay- medication called nitisinone (previously called Lac-St-Jean region of Quebec. -

Orfadin® (Nitisinone) P&T Approval Date 5/2016, 5/2017, 5/2018, 5/2019, 5/2020, 5/2021 Effective Date 8/1/2021; Oxford Only: 8/1/2021
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2021 P 1185-6 Program Prior Authorization/Notification Medication Orfadin® (nitisinone) P&T Approval Date 5/2016, 5/2017, 5/2018, 5/2019, 5/2020, 5/2021 Effective Date 8/1/2021; Oxford only: 8/1/2021 1. Background: Orfadin (nitisinone) is a hydroxy-phenylpyruvate dioxygenase inhibitor indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 (HT-1) in combination with dietary restriction of tyrosine and phenylalanine. Coverage for Orfadin will be provided for patients who meet the following criteria: 2. Coverage Criteria: A. Initial Authorization 1. Orfadin will be approved based on the following criteria: a. Diagnosis of hereditary tyrosinemia type 1 AND b. Orfadin is being used as an adjunct to diet modification. Authorization will be issued for 12 months. B. Reauthorization 1. Orfadin will be approved based on the following criterion: a. Patient shows evidence of positive clinical response (e.g., decrease in urinary/plasma succinylacetone and alpha-1-microglobulin levels) while on Orfadin therapy Authorization will be issued for 24 months. 3. Additional Clinical Rules: • Notwithstanding Coverage Criteria, UnitedHealthcare may approve initial and re- authorization based solely on previous claim/medication history, diagnosis codes (ICD-10) and/or claim logic. Use of automated approval and re-approval processes varies by program and/or therapeutic class. • Supply limits may be in place. © 2021 UnitedHealthcare Services, Inc. 1 4. References: 1. Orfadin [prescribing information]. Waltham, MA. Sobi, Inc. May 2019. Program Prior Authorization/Notification – Orfadin (nitisinone) capsules, for oral use, and oral suspension Change Control 5/2016 New program 5/2017 Annual review.