Balanced Coagulopathy of Liver Failure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crofab Brochure
Control With Confidence The only antivenom derived from native US pit vipers to treat envenomations from all species of North American pit vipers1 CroFab is the only antivenom Derived from geographically and clinically relevant US snakes for comprehensive coverage of all North American pit viper envenomations1 Designed with small, venom-specific protein (Fab) fragments for rapid neutralization of venom toxins throughout affected tissue1,2 With Level 1 evidence in the treatment of copperhead envenomation3 Manufactured to yield the highest level of quality, purity, and safety1 With a proven efficacy and safety profile, backed by >20 years of clinical experience1 Reliably supplied throughout the United States4 CroFab meets World Health Organization (WHO) guidelines for effective antivenom, utilizing venom from 4 clinically relevant pit viper species native to the United States.1,5 Indication CroFab® Crotalidae Polyvalent Immune Fab (Ovine) is a sheep-derived antivenin indicated for the management of adult and pediatric patients with North American crotalid envenomation. The term crotalid is used to describe the Crotalinae subfamily (formerly known as Crotalidae) of venomous snakes which includes rattlesnakes, copperheads and cottonmouths/water moccasins. Important Safety Information Contraindications Do not administer CroFab® to patients with a known history of hypersensitivity to any of its components, or to papaya or papain unless the benefits outweigh the risks and appropriate management for anaphylactic reactions is readily available. Warnings and Precautions Coagulopathy: In clinical trials, recurrent coagulopathy (the return of a coagulation abnormality after it has been successfully treated with antivenin), characterized by decreased fibrinogen, decreased platelets, and elevated prothrombin time, occurred in approximately half of the patients studied; one patient required re-hospitalization and additional antivenin administration. -

Newborn Screening Laboratory Manual of Services
Newborn Screening Laboratory Manual of Services Test Panel: Please see the following links for a detailed description of testing in the Newborn Screening section. Information about the Newborn Screening program is available here. Endocrine Disorders Congenital adrenal hyperplasia (CAH) Congenital hypothyroidism (TSH) Hemoglobinopathies Sickle cell disease (FS) Alpha (Barts) Sickle βeta Thalassemia (FSA) Other sickling hemoglobinopathies such as: FAS FAC FAD FAE Homozygous conditions such as: FC FD FE Metabolic Disorders Biotinidase deficiency Galactosemia Cystic fibrosis (CF) first tier screening for elevated immunoreactive trypsinogen (IRT) Cystic fibrosis second tier genetic mutation analysis on the top 4% IRT concentrations. Current alleles detected : F508del, I507del, G542X, G85E, R117H, 621+1G->T, 711+1G->T, R334W, R347P, A455E, 1717-1G->A, R560T, R553X, G551D, 1898+1G->A, 2184delA, 2789+5G->A, 3120+1G->A, R1162X, 3659delC, 3849+10kbC->T, W1282X, N1303K, IVS polyT T5/T7/T9 *Currently validating a mutation panel that includes the above alleles in addition to the following: 1078delT, Y122X, 394delTT, R347H, M1101K, S1255X, 1898+5G->T, 2183AA->G, 2307insA, Y1092X, 3876delA, 3905insT, S549N, S549R_1645A->C, S549R-1647T->G, S549R-1647T->G, V520F, A559T, 1677delTA, 2055del9->A, 2143delT, 3199del6, 406-1G->A, 935delA, D1152H, CFTRdele2, E60X, G178R, G330X, K710X, L206W, Q493X, Q890X, R1066C, R1158X, R75X, S1196X, W1089X, G1244E, G1349D, G551S, R560KT, S1251N, S1255P Amino acid disorders Phenylketonuria (PKU) / Hyperphenylalaninemia Maple -

TYR I, II, III Act Sheet
Newborn Screening ACT Sheet Increased Tyrosine Tyrosinemia Differential Diagnosis: Tyrosinemia I (hepatorenal); Tyrosinemia II (oculocutaneous); Tyrosinemia III; transient hypertyrosinemia; liver disease. Condition Description: In the hepatorenal form, tyrosine from ingested protein and phenylalanine metabolism cannot be metabolized by fumarylacetoacetate hydrolase to fumaric acid and acetoacetic acid. The resulting fumarylacetoacetate accumulates and is converted to succinylacetone, the diagnostic metabolite, which is liver toxic, and leads to elevated tyrosine. Tyrosinemias II and III are due to other defects in tyrosine degradation. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: plasma quantitative amino acids; urine succinylacetone and liver function tests. • Repeat newborn screen if the second screen has not been done. • Provide family with basic information about tyrosinemia. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino acid analysis will show increased tyrosine in all of the tyrosinemias. Urine organic acid analysis may reveal increased succinylacetone in Tyrosinemia I. Clinical Considerations: Tyrosinemia I is usually asymptomatic in the neonate. If untreated, it will cause liver disease and cirrhosis -
Download This PDF File
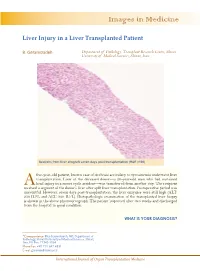
Images in Medicine Liver Injury in a Liver Transplanted Patient B. Geramizadeh Department of Pathology, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Sections from liver allograft seven days post-transplantation (H&E ×100) five-year-old patient, known case of cirrhosis secondary to tyrosinemia underwent liver transplantation. Liver of the deceased donor—a 20-year-old man who had sustained A head injury in a motor cycle accident—was transferred from another city. The recipient received a segment of the donor’s liver after split liver transplantation. Postoperative period was uneventful. However, seven days post-transplantation, the liver enzymes were still high (ALT: 250 IU/L and ALT: 320 IU/L). Histopathologic examination of the transplanted liver biopsy is shown in the above photomicrograph. The patient improved after two weeks and discharged from the hospital in good condition. WHAT IS YOUR DIAGNOSIS? *Correspondence: Bita Geramizadeh, MD, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran. PO Box: 71345-1864 Phone/Fax: +98-711-647-4331 E-mail: [email protected] International Journal of Organ Transplantation Medicine B. Geramizadeh DIAGNOSIS: PRESERVATION/REPERFUSION INJURY dvances in organ preservation have duct obstruction. The distinctive pattern of reduced preservation injury. Neverthe- bile ductular cholestasis is that it is usually as- Aless, when storage time exceeds 10 to sociated with sepsis. Drug toxicity can mimic 12 hours, post-transplantation complications, every change in the liver and should always be due to preservation/reperfusion injury, be- excluded [5]. come more common [1]. In our patient, prolonged cold ischemic time Ischemic injury to the graft is divided into (transfer of the liver from another city) and cold ischemia—secondary to prolonged pres- probably small for size graft (split liver trans- ervation—and warm ischemia, which occurs plantation) were predisposing factors. -

What Disorders Are Screened for by the Newborn Screen?
What disorders are screened for by the newborn screen? Endocrine Disorders The endocrine system is important to regulate the hormones in our bodies. Hormones are special signals sent to various parts of the body. They control many things such as growth and development. The goal of newborn screening is to identify these babies early so that treatment can be started to keep them healthy. To learn more about these specific disorders please click on the name of the disorder below: English: Congenital Adrenal Hyperplasia Esapnol Hiperplasia Suprarrenal Congenital - - http://www.newbornscreening.info/Parents/otherdisorders/CAH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CAH.html - Congenital Hypothyroidism (Hipotiroidismo Congénito) - http://www.newbornscreening.info/Parents/otherdisorders/CH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CH.html Hematologic Conditions Hemoglobin is a special part of our red blood cells. It is important for carrying oxygen to the parts of the body where it is needed. When people have problems with their hemoglobin they can have intense pain, and they often get sick more than other children. Over time, the lack of oxygen to the body can cause damage to the organs. The goal of newborn screening is to identify babies with these conditions so that they can get early treatment to help keep them healthy. To learn more about these specific disorders click here (XXX). - Sickle Cell Anemia (Anemia de Célula Falciforme) - http://www.newbornscreening.info/Parents/otherdisorders/SCD.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/SCD.html - SC Disease (See Previous Link) - Sickle Beta Thalassemia (See Previous Link) Enzyme Deficiencies Enzymes are special proteins in our body that allow for chemical reactions to take place. -

Haemostatic Problems in Liver Disease
Gut: first published as 10.1136/gut.27.3.339 on 1 March 1986. Downloaded from Gut, 1986, 27, 339-349 Progress report Haemostatic problems in liver disease The liver plays a major role in the control of coagulation and as a result haemostatic problems are detected in approximately 75% of patients with liver disease.1 The coagulation abnormalities are both complex and multifactorial and depend on the balance between hepatic synthesis and clearance of activated coagulation proteins and their inhibitors; the presence or absence of dysfibrinogenaemia; thrombocytopenia, abnormal platelet function, and disseminated intravascular coagulation. Some patients will present with petechiae, ecchymosis or epistaxis, but most patients are asymptomatic or only bleed after venepuncture or liver biopsy. Alternatively haemorrhage may be life threatening and patients may die from variceal bleeding or from disseminated intravascular coagulation. The reasons for this disparity are not yet clear, but after the introduction of newer techniques, in particular the development of immunological assays for the antigens of coagulation proteins, our understanding of these problems has improved. The normal coagulation and fibrinolytic systems are depicted in Figures 1 and 2 while the major .__Intrinsic___ _ pathwY http://gut.bmj.com/ Kallikrein.o- PK | HMWKq 8t XII -*xiiXIIa_4------- ATIII ~ ~ 'I L1HMWK - XI* Xla %' xC-a; --------- -- on September 28, 2021 by guest. Protected copyright. IX - IXa VII -e'VIIca Extrinsic pathway [X VIII a Ce X P'okin C ATIII Ca+ XIII Common mI ~V PL II a pathway I XIIIa Fibrinogen - Fibrin Fig. 1 The coagulation cascade. HMWK=high molecular weight Kinogen, PK=Pre-Kallikrein, A TIII=antithrornbin III, PL=platelets, Ca" = Calcium, TF=tissue factor, -t- =proteolytic activation, -+=conversion ofcoagulation protein, -- -+=inhibition by plasma inhibitors, tit =crosslinking, a=activated coagulation enzyme. -

The Underrecognized Prothrombotic Vascular Disease of COVID-19
Journal Articles 2020 The underrecognized prothrombotic vascular disease of COVID-19. KP Cohoon G Mahé AC Spyropoulos Zucker School of Medicine at Hofstra/Northwell, [email protected] Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Internal Medicine Commons Recommended Citation Cohoon K, Mahé G, Spyropoulos A. The underrecognized prothrombotic vascular disease of COVID-19.. 2020 Jan 01; 4(5):Article 6487 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/ 6487. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. For more information, please contact [email protected]. Received: 6 May 2020 | Revised: 16 May 2020 | Accepted: 21 May 2020 DOI: 10.1002/rth2.12396 LETTER TO THE EDITOR The underrecognized prothrombotic vascular disease of COVID-19 We have read with interest “COVID-19-associated coagulopathy around elevated markers of hypercoagulability, including D-dimer, and thromboembolic disease: Commentary on an interim expert tissue factor expression, fibrinogen levels, factor VIII levels, guidance” recently provided by Cannegieter and Klok.1 This com- short-activated partial thromboplastin time, platelet binding, and mentary exemplifies the importance that venous thromboembolism thrombin formation.8 Based on well-defined clinical and laboratory (VTE) and atheroembolism may be underrepresented and a cause parameters, a proposal for staging COVID-19 coagulopathy may for increased morbidity and mortality among coronavirus disease provide treatment algorithms stratified into 3 stages.9 However, 2019 (COVID-19) patients. -

Guidelines for the Management of Haemophilia in Australia
Guidelines for the management of haemophilia in Australia A joint project between Australian Haemophilia Centre Directors’ Organisation, and the National Blood Authority, Australia © Australian Haemophilia Centre Directors’ Organisation, 2016. With the exception of any logos and registered trademarks, and where otherwise noted, all material presented in this document is provided under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Australia (http://creativecommons.org/licenses/by-nc-sa/3.0/au/) licence. You are free to copy, communicate and adapt the work for non-commercial purposes, as long as you attribute the authors and distribute any derivative work (i.e. new work based on this work) only under this licence. If you adapt this work in any way or include it in a collection, and publish, distribute or otherwise disseminate that adaptation or collection to the public, it should be attributed in the following way: This work is based on/includes the Australian Haemophilia Centre Directors’ Organisation’s Guidelines for the management of haemophilia in Australia, which is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Australia licence. Where this work is not modified or changed, it should be attributed in the following way: © Australian Haemophilia Centre Directors’ Organisation, 2016. ISBN: 978-09944061-6-3 (print) ISBN: 978-0-9944061-7-0 (electronic) For more information and to request permission to reproduce material: Australian Haemophilia Centre Directors’ Organisation 7 Dene Avenue Malvern East VIC 3145 Telephone: +61 3 9885 1777 Website: www.ahcdo.org.au Disclaimer This document is a general guide to appropriate practice, to be followed subject to the circumstances, clinician’s judgement and patient’s preferences in each individual case. -

Thrombosis and Coagulopathy Guidance in COVID-19
Thrombosis and Coagulopathy Guidance in COVID-19 The risk of thrombosis in COVID-19 Patients with COVID-19 are at risk of venous thromboembolism (VTE), which is a deep vein thrombosis (DVT) or pulmonary embolism (PE). It is still unknown if this risk is higher in comparison to non-COVID acutely ill patients. How to interpret a D-dimer level in COVID-19 An elevated or rising D-dimer level is commonly seen in patients with COVID-19 (~50%) and is because of a profound inflammatory state. An elevated D-dimer alone does not warrant investigation for VTE unless there is also a high clinical suspicion for DVT and/or PE. Pulmonary embolism should be considered in admitted patients with COVID-19 who have unexplained worsening respiratory status/hypoxia, unexplained hypotension or tachycardia, or signs of DVT. If the D-dimer is normal, this has the ability to rule out VTE. Although the false negative rate of D-dimer testing (i.e. DVT/PE is present but the result is normal) is unknown in COVID-19 patients, low rates of 1- 2% using highly sensitive D-dimer assays have been reported in other high risk populations. Therefore, a normal level D-dimer level provides reasonable confidence that VTE is not present. Prevention of thrombosis All hospitalized patients with suspected or confirmed COVID-19 should receive pharmacologic thromboprophylaxis, preferably with low-molecular-weight heparin (LMWH). LMWH prophylaxis should be held if the patient is bleeding or has a platelet count <30 x 109/L. In patients where anticoagulation is contraindicated, use mechanical thromboprophylaxis (e.g. -
![PROTEIN C DEFICIENCY 1215 Adulthood and a Large Number of Children and Adults with Protein C Mutations [6,13]](https://docslib.b-cdn.net/cover/8040/protein-c-deficiency-1215-adulthood-and-a-large-number-of-children-and-adults-with-protein-c-mutations-6-13-1348040.webp)
PROTEIN C DEFICIENCY 1215 Adulthood and a Large Number of Children and Adults with Protein C Mutations [6,13]
Haemophilia (2008), 14, 1214–1221 DOI: 10.1111/j.1365-2516.2008.01838.x ORIGINAL ARTICLE Protein C deficiency N. A. GOLDENBERG* and M. J. MANCO-JOHNSON* *Hemophilia & Thrombosis Center, Section of Hematology, Oncology, and Bone Marrow Transplantation, Department of Pediatrics, University of Colorado Denver and The ChildrenÕs Hospital, Aurora, CO; and Division of Hematology/ Oncology, Department of Medicine, University of Colorado Denver, Aurora, CO, USA Summary. Severe protein C deficiency (i.e. protein C ment of acute thrombotic events in severe protein C ) activity <1 IU dL 1) is a rare autosomal recessive deficiency typically requires replacement with pro- disorder that usually presents in the neonatal period tein C concentrate while maintaining therapeutic with purpura fulminans (PF) and severe disseminated anticoagulation; protein C replacement is also used intravascular coagulation (DIC), often with concom- for prevention of these complications around sur- itant venous thromboembolism (VTE). Recurrent gery. Long-term management in severe protein C thrombotic episodes (PF, DIC, or VTE) are common. deficiency involves anticoagulation with or without a Homozygotes and compound heterozygotes often protein C replacement regimen. Although many possess a similar phenotype of severe protein C patients with severe protein C deficiency are born deficiency. Mild (i.e. simple heterozygous) protein C with evidence of in utero thrombosis and experience deficiency, by contrast, is often asymptomatic but multiple further events, intensive treatment and may involve recurrent VTE episodes, most often monitoring can enable these individuals to thrive. triggered by clinical risk factors. The coagulopathy in Further research is needed to better delineate optimal protein C deficiency is caused by impaired inactiva- preventive and therapeutic strategies. -

Tyrosinemia (Type I) – Amino Acid Disorder
Tyrosinemia (Type I) – Amino Acid Disorder What are amino acid disorders? Kidney disease may lead to rickets, a bone The amino acid disorders are a class of disease. The nerves may also be affected. inherited metabolic conditions that occur when Some babies may have a rapid heart rate, certain amino acids either cannot be broken breathing difficulties, and seizures. down or cannot be produced by the body, Occasionally, individuals with liver damage resulting in the toxic accumulation of some have a higher risk of developing liver cancer. substances and the deficiency of other Acute liver and kidney damage can lead to substances. death. What is tyrosinemia? How is the diagnosis confirmed? In tyrosinemia, the amino acid tyrosine cannot The diagnosis is confirmed by measuring the be broken down properly, leading to a toxic levels of amino acids in the blood and organic accumulation of this amino acid and its acids in the urine. The finding of metabolites in the body. succinylacetone in the urine is diagnostic. Enzyme testing and genetic testing of the FAH What is its incidence? gene may also be used to confirm the Tyrosinemia affects about 1 out every 100,000 diagnosis. Diagnostic testing is arranged by babies born in BC. Although tyrosinemia specialists at BC Children’s Hospital. occurs in all ethnic groups, it is more common in certain populations. Its incidence has been What is the treatment of the disease? reported as high as 1 in 2,000 in the French Children with tyrosinemia are treated with a Canadian population living in the Saguenay- medication called nitisinone (previously called Lac-St-Jean region of Quebec. -
Canine and Feline Coagulopathies Office News Michelle Fulks, DVM, Virginia Sinnott, DVM, DACVECC William B
Monthly Update August 2013 Issue Contributors: Michelle Fulks, DVM, Virginia Sinnott, DVM, DACVECC, William B. Henry DVM, DACVS Editor: William B. Henry DVM, DACVS Canine and Feline Coagulopathies Office News Michelle Fulks, DVM, Virginia Sinnott, DVM, DACVECC William B. Henry, Jr. DVM, DACVS Canine and Feline Coagulopathies Bleeding disorders are considered a potential life-threatening emergency in small animal practice. It is crucial to recognize the potential for a coagulation disorder through history and physical exam findings, pursue appropriate diagnostic tests and then treat appropriately in order to prevent massive bleeding in these patients. Three areas of the hemostatic system may be affected to cause coagulopathies: Amanda Spencer, CVT joined our surgery team in late 2012. She has 10 1. Disorders of Primary Hemostasis years experience in surgery and 2. Disorders of Secondary Hemostasis emergency care. Her sole focus at BVS 3. Disorders of Fibrinolysis is with the surgical practice. Her skills, dedication to excellence, and caring Primary hemostasis is the formation of the initial platelet plug. Decreases in attitude towards our clients and platelet number, platelet function, or reduced von Willebrand factor (VWF) can all patients, has been outstanding. Her cause disorders of primary hemostasis and lead to mucosal bleeding or calm upbeat personality makes our bruising. Secondary hemostasis is the formation of a stable fibrin clot via cascade days together as a team more of enzymes that ultimately convert fibrinogen to fibrin. Defects in coagulation enjoyable. She is one of those staff factors can lead to severe bleeding diatheses. Fibrinolysis is the breakdown of the members "behind the public eye" who fibrin clot by plasmin.