Bonytongues to Herrings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

Download Animal List
MICHIGAN'S SPECIAL ANIMALS Endangered, Threatened, Special Concern, and Probably Extirpated This list presents the Endangered (E), Threatened (T), and Probably Extirpated (X) animal species of Michigan, which are protected under the Endangered Species Act of the State of Michigan (Part 365 of PA 451, 1994 Michigan Natural Resources and Environmental Protection Act). The current list became effective on April 9, 2009, after extensive review by technical advisors to the Michigan Department of Natural Resources and the citizenry of the state. Also included in this list are animal species of Special Concern (SC). While not afforded legal protection under the Act, many of these species are of concern because of declining or relict populations in the state. Should these species continue to decline, they would be recommended for Threatened or Endangered status. Protection of Special Concern species now, before they reach dangerously low population levels, would prevent the need to list them in the future by maintaining adequate numbers of self-sustaining populations within Michigan. Some other potentially rare species are listed as Special Concern pending more precise information on their status in the state; when such information becomes available, they could be moved to threatened or endangered status or deleted from the list. This list was produced by the Endangered Species Program of the Michigan Department of Natural Resources and the Michigan Natural Features Inventory. English names in common usage or from published sources have been incorporated, when possible, to promote public understanding of and participation in the Endangered Species Program. To comment on the list or request additional copies, or for information on the Endangered Species Program, contact the Endangered Species Coordinator, Wildlife Division, Michigan Department of Natural Resources, P.O. -

V a Tion & Management of Reef Fish Sp a Wning Aggrega Tions
handbook CONSERVATION & MANAGEMENT OF REEF FISH SPAWNING AGGREGATIONS A Handbook for the Conservation & Management of Reef Fish Spawning Aggregations © Seapics.com Without the Land and the Sea, and their Bounties, the People and their Traditional Ways would be Poor and without Cultural Identity Fijian Proverb Why a Handbook? 1 What are Spawning Aggregations? 2 How to Identify Spawning Aggregations 2 Species that Aggregate to Spawn 2 Contents Places Where Aggregations Form 9 Concern for Spawning Aggregations 10 Importance for Fish and Fishermen 10 Trends in Exploited Aggregations 12 Managing & Conserving Spawning Aggregations 13 Research and Monitoring 13 Management Options 15 What is SCRFA? 16 How can SCRFA Help? 16 SCRFA Work to Date 17 Useful References 18 SCRFA Board of Directors 20 Since 2000, scientists, fishery managers, conservationists and politicians have become increasingly aware, not only that many commercially important coral reef fish species aggregate to spawn (reproduce) but also that these important reproductive gatherings are particularly susceptible to fishing. In extreme cases, when fishing pressure is high, aggregations can dwindle and even cease to form, sometimes within just a few years. Whether or not they will recover and what the long-term effects on the fish population(s) might be of such declines are not yet known. We do know, however, that healthy aggregations tend to be associated with healthy fisheries. It is, therefore, important to understand and better protect this critical part of the life cycle of aggregating species to ensure that they continue to yield food and support livelihoods. Why a Handbook? As fishing technology improved in the second half of the twentieth century, engines came to replace sails and oars, the cash economy developed rapidly, and human populations and demand for seafood grew, the pressures on reef fishes for food, and especially for money, increased enormously. -

The Need for Sustainable Management of Coral Reef Fish Spawning Aggregations
Secretariat of the Pacific Community Seventh Heads of Fisheries Meeting (28 Feb.–4 March 2011, Noumea, New Caledonia) Working Paper 7 Original: English The need for sustainable management of coral reef fish spawning aggregations Yvonne Sadovy PhD The University of Hong-Kong and Eric Clua Coral Reef Initiative for the South Pacific (CRISP), SPC www.spc.int/fame/ SPC/HOF7/Working Paper 7 Page 1 The need for sustainable management of coral reef fish spawning aggregations 1. Many commercially important species of reef fishes exhibit the habit of ‘aggregation-spawning’ whereby all mating takes place in large temporary gatherings of conspecifics. Sometimes fish also migrate in large numbers to spawning sites. These mating-related gatherings and movements are often highly predictable in time and location and hence, once discovered, are often the basis of important seasonal fisheries. Yet, as we have come to discover over the last two decades, such reproductive gatherings are highly vulnerable to over-fishing and, if severely compromised by fishing, may cease to form completely. If this happens adults will no longer produce the eggs and larvae that form the basis of fisheries at non-reproductive times of the year, and the fishery is likely to collapse. Collapse would represent the loss of significant fisheries with important and sometimes serious implications for the human communities that depend on such fishes. Moreover, aggregations can represent significant biomass and movements of animals, and are formed by many species, highlighting the ecosystem importance of these biological events. 2. Species that predictably aggregate to spawn, or gather in large spawning migrations, include a diverse range of fishes of much importance as food and commercial benefit in the Pacific. -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -

Illinois Fishes Volume 1 Poster
Illinois Photo © 2010, T. Lawrence, Great Lakes Fishery Commission detail of sea lamprey's oral disc FFiisshheess detail of mooneye teeth sea lamprey attached to lake trout Salvelinus namaycush Petromyzon marinus mooneye Hiodon tergisus Photo © 2010, Great Lakes Fishery Commission Volume I Photos © 2010, Uland Thomas chestnut lamprey Ichthyomyzon castaneus goldeye Hiodon alosoides Photo © 2010, Philip Willink/The Field Museum of Natural History, Chicago Photo © 2010, Shedd Aquarium detail of silver lamprey's oral disc silver lamprey Ichthyomyzon unicuspis northern brook lamprey Ichthyomyzon fossor Photo © 2010, Shedd Aquarium Photo © 2010, Philip Willink/The Field Museum of Natural History, Chicago Alabama shad Alosa alabamae Photo © 2010, Patrick O’Neil, Geological Survey of Alabama least brook lamprey Lampetra aepyptera American brook lamprey Lampetra lamottei Photo © 2010, Julie Zimmerman Photo © 2010, Philip Willink/The Field Museum of Natural History, Chicago skipjack herring Alosa chrysochloris Photo © 2010, Patrick O’Neil, Geological Survey of Alabama alewife Alosa pseudoharengus Photo © 2010, Engbretson Underwater Photography shovelnose sturgeon Scaphirhynchus platorynchus Photo © 2010, Shedd Aquarium bowfin Amia calva Photo © 2010, Engbretson Underwater Photography gizzard shad Dorosoma cepedianum American eel Anguilla rostrata Photo © 2010, Shedd Aquarium Photo © 2010, Engbretson Underwater Photography pallid sturgeon Scaphirhynchus albus shortnose gar Lepisosteus platostomus threadfin shad Dorosoma petenense Photo © 2010, NEBRASKAland Magazine/Nebraska Game and Parks Commission Photo © 2010, Uland Thomas Photo © 2010, Uland Thomas lake sturgeon Acipenser fulvescens paddlefish Polyodon spathula Photo © 2010, Engbretson Underwater Photography Photo © 2010, Engbretson Underwater Photography longnose gar Lepisosteus osseus spotted gar Lepisosteus oculatus Photo © 2010, Engbretson Underwater Photography Photo © 2010, Uland Thomas pproximately 22,000 fish species inhabit the Species List Species are not shown in proportion to actual size. -

A Review of the Systematic Biology of Fossil and Living Bony-Tongue Fishes, Osteoglossomorpha (Actinopterygii: Teleostei)
Neotropical Ichthyology, 16(3): e180031, 2018 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20180031 Published online: 11 October 2018 (ISSN 1982-0224) Copyright © 2018 Sociedade Brasileira de Ictiologia Printed: 30 September 2018 (ISSN 1679-6225) Review article A review of the systematic biology of fossil and living bony-tongue fishes, Osteoglossomorpha (Actinopterygii: Teleostei) Eric J. Hilton1 and Sébastien Lavoué2,3 The bony-tongue fishes, Osteoglossomorpha, have been the focus of a great deal of morphological, systematic, and evolutio- nary study, due in part to their basal position among extant teleostean fishes. This group includes the mooneyes (Hiodontidae), knifefishes (Notopteridae), the abu (Gymnarchidae), elephantfishes (Mormyridae), arawanas and pirarucu (Osteoglossidae), and the African butterfly fish (Pantodontidae). This morphologically heterogeneous group also has a long and diverse fossil record, including taxa from all continents and both freshwater and marine deposits. The phylogenetic relationships among most extant osteoglossomorph families are widely agreed upon. However, there is still much to discover about the systematic biology of these fishes, particularly with regard to the phylogenetic affinities of several fossil taxa, within Mormyridae, and the position of Pantodon. In this paper we review the state of knowledge for osteoglossomorph fishes. We first provide an overview of the diversity of Osteoglossomorpha, and then discuss studies of the phylogeny of Osteoglossomorpha from both morphological and molecular perspectives, as well as biogeographic analyses of the group. Finally, we offer our perspectives on future needs for research on the systematic biology of Osteoglossomorpha. Keywords: Biogeography, Osteoglossidae, Paleontology, Phylogeny, Taxonomy. Os peixes da Superordem Osteoglossomorpha têm sido foco de inúmeros estudos sobre a morfologia, sistemática e evo- lução, particularmente devido à sua posição basal dentre os peixes teleósteos. -

Rhodopsin Gene Evolution in Early Teleost Fishes
RESEARCH ARTICLE Rhodopsin gene evolution in early teleost fishes 1 2 1 Jhen-Nien Chen , Sarah Samadi , Wei-Jen ChenID * 1 Institute of Oceanography, National Taiwan University, Taipei, Taiwan, 2 Institute de SysteÂmatique, E volution, Biodiversite (ISYEB), MuseÂum National d'Histoire Naturelle±CNRS, Sorbonne UniversiteÂ, EPHE, Paris, France * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Rhodopsin mediates an essential step in image capture and is tightly associated with visual adaptations of aquatic organisms, especially species that live in dim light environments (e.g., the deep sea). The rh1 gene encoding rhodopsin was formerly considered a single- copy gene in genomes of vertebrates, but increasing exceptional cases have been found in teleost fish species. The main objective of this study was to determine to what extent the OPEN ACCESS visual adaptation of teleosts might have been shaped by the duplication and loss of rh1 Citation: Chen J-N, Samadi S, Chen W-J (2018) genes. For that purpose, homologous rh1/rh1-like sequences in genomes of ray-finned Rhodopsin gene evolution in early teleost fishes. PLoS ONE 13(11): e0206918. https://doi.org/ fishes from a wide taxonomic range were explored using a PCR-based method, data mining 10.1371/journal.pone.0206918 of public genetic/genomic databases, and subsequent phylogenomic analyses of the Editor: Michael Schubert, Laboratoire de Biologie retrieved sequences. We show that a second copy of the fish-specific intron-less rh1 is pres- du DeÂveloppement de Villefranche-sur-Mer, ent in the genomes of most anguillids (Elopomorpha), Hiodon alosoides (Osteoglossomor- FRANCE pha), and several clupeocephalan lineages. -

Teleost Radiation Teleost Monophyly
Teleost Radiation Teleostean radiation - BIG ~ 20,000 species. Others put higher 30,000 (Stark 1987 Comparative Anatomy of Vertebrates) About 1/2 living vertebrates = teleosts Tetrapods dominant land vertebrates, teleosts dominate water. First = Triassic 240 my; Originally thought non-monophyletic = many independent lineages derived from "pholidophorid" ancestry. More-or-less established teleostean radiation is true monophyletic group Teleost Monophyly • Lauder and Liem support this notion: • 1. Mobile premaxilla - not mobile like maxilla halecostomes = hinged premaxilla modification enhancing suction generation. Provides basic structural development of truly mobile premaxilla, enabling jaw protrusion. Jaw protrusion evolved independently 3 times in teleostean radiation 1) Ostariophysi – Cypriniformes; 2) Atherinomorpha and 2) Percomorpha – especially certain derived percomorphs - cichlids and labroid allies PREMAXILLA 1 Teleost Monophyly • Lauder & Liem support notion: • 2. Unpaired basibranchial tooth plates (trend - consolidation dermal tooth patches in pharynx). • Primitive = whole bucco- pharynx w/ irregular tooth patches – consolidate into functional units - modified w/in teleostei esp. functional pharyngeal jaws. Teleost Monophyly • Lauder and Liem support this notion: • 3. Internal carotid foramen enclosed in parasphenoid (are all characters functional, maybe don't have one - why should they?) 2 Teleost Tails • Most interesting structure in teleosts is caudal fin. • Teleosts possess caudal skeleton differs from other neopterygian fishes - Possible major functional significance in Actinopterygian locomotor patterns. • Halecomorphs-ginglymodes = caudal fin rays articulate with posterior edge of haemal spines and hypurals (modified haemal spines). Fin is heterocercal (inside and out). Ginglymod - Gars Halecomorph - Amia 3 Tails • “Chondrostean hinge” at base of upper lobe - weakness btw body and tail lobe. Asymmetrical tail = asymmetrical thrust with respect to body axis. -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people. -
Field Identification Guide to the Living Marine Resources in Kenya
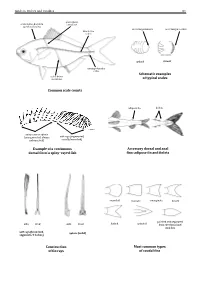
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery.