WO 2013/063263 Al May 2013 (02.05.2013) W P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Considerations in Perioperative Assessment of Valproic Acid Coagulopathy Claude Abdallah George Washington University
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Anesthesiology and Critical Care Medicine Faculty Anesthesiology and Critical Care Medicine Publications 1-2014 Considerations in perioperative assessment of valproic acid coagulopathy Claude Abdallah George Washington University Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/smhs_anesth_facpubs Part of the Anesthesia and Analgesia Commons APA Citation Abdallah, C. (2014). Considerations in perioperative assessment of valproic acid coagulopathy. Journal of Anaesthesiology Clinical Pharmacology, Volume 30, Issue 1 (). http://dx.doi.org/10.4103/0970-9185.125685 This Journal Article is brought to you for free and open access by the Anesthesiology and Critical Care Medicine at Health Sciences Research Commons. It has been accepted for inclusion in Anesthesiology and Critical Care Medicine Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. [Downloaded free from http://www.joacp.org on Tuesday, February 25, 2014, IP: 128.164.86.61] || Click here to download free Android application for this journal Revv iew Article Considerations in perioperative assessment of valproic acid coagulopathy Claude Abdallah Department of Anesthesiology, Children’s National Medical Center, The George Washington University Medical Center, NW Washington, DC, USA Abstract Valproic acid (VPA) is one of the widely prescribed antiepileptic drugs in children with multiple indications. VPA-induced coagulopathy may occur and constitute a pharmacological and practical challenge affecting pre-operative evaluation and management of patients receiving VPA therapy. This review summarizes the different studies documenting the incidence, severity and available recommendations related to this adverse effect. -
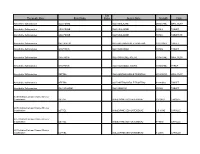
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

(12) Patent Application Publication (10) Pub. No.: US 2011/0244057 A1 Ehrenberg (43) Pub
US 2011024.4057A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0244057 A1 Ehrenberg (43) Pub. Date: Oct. 6, 2011 (54) COMBINATION THERAPIES WITH Related U.S. Application Data TOPIRAMATE FOR SEIZURES, RESTLESS LEGS SYNDROME, AND OTHER (60) Provisional application No. 61/100,232, filed on Sep. NEUROLOGICAL CONDITIONS 25, 2008. Publication Classification (51) Int. Cl. (76) Inventor: Bruce L. Ehrenberg, New York, A633/26 (2006.01) NY (US) A63L/35 (2006.01) A633/06 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/120,933 A6IP 25/28 (2006.01) A6IP 25/08 (2006.01) (22) PCT Filed: Sep. 25, 2009 (52) U.S. Cl. .......... 424/648: 514/454; 424/682; 424/646 (57) ABSTRACT (86). PCT No.: PCT/US2009/058495 The invention provides compositions and methods of treating various conditions, including neurological conditions, with S371 (c)(1), pharmaceutical compositions including a sulfamate (e.g., (2), (4) Date: Jun. 9, 2011 topiramate) and magnesium. US 2011/024.4057 A1 Oct. 6, 2011 COMBINATION THERAPES WITH TOPIRAMATE FOR SEIZURES, RESTLESS LEGS SYNDROME, AND OTHER II NEUROLOGICAL CONDITIONS CROSS-REFERENCE TO RELATED APPLICATIONS 0001. This application claims the benefit of the priority date of U.S. Application No. 61/100,232, filed Sep. 25, 2008. For the purpose of any U.S. patent that may issue from the 0005 wherein RandR, are the same or different and are U.S. national phase correlate of the present application, the hydrogen, lower alkyl or are alkyl and are joined to form a content of the priority document is hereby incorporated by cyclopentyl or cyclohexyl ring, and (b) magnesium. -
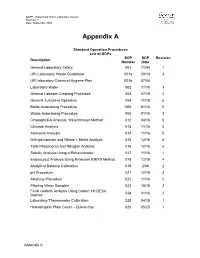
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Pharmacokinetic Drug–Drug Interactions Among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients
International Journal of Molecular Sciences Review Pharmacokinetic Drug–Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients Marta Kara´zniewicz-Łada 1 , Anna K. Główka 2 , Aniceta A. Mikulska 1 and Franciszek K. Główka 1,* 1 Department of Physical Pharmacy and Pharmacokinetics, Poznan University of Medical Sciences, 60-781 Pozna´n,Poland; [email protected] (M.K.-Ł.); [email protected] (A.A.M.) 2 Department of Bromatology, Poznan University of Medical Sciences, 60-354 Pozna´n,Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-(0)61-854-64-37 Abstract: Anti-epileptic drugs (AEDs) are an important group of drugs of several generations, rang- ing from the oldest phenobarbital (1912) to the most recent cenobamate (2019). Cannabidiol (CBD) is increasingly used to treat epilepsy. The outbreak of the SARS-CoV-2 pandemic in 2019 created new challenges in the effective treatment of epilepsy in COVID-19 patients. The purpose of this review is to present data from the last few years on drug–drug interactions among of AEDs, as well as AEDs with other drugs, nutrients and food. Literature data was collected mainly in PubMed, as well as google base. The most important pharmacokinetic parameters of the chosen 29 AEDs, mechanism of action and clinical application, as well as their biotransformation, are presented. We pay a special attention to the new potential interactions of the applied first-generation AEDs (carba- Citation: Kara´zniewicz-Łada,M.; mazepine, oxcarbazepine, phenytoin, phenobarbital and primidone), on decreased concentration Główka, A.K.; Mikulska, A.A.; of some medications (atazanavir and remdesivir), or their compositions (darunavir/cobicistat and Główka, F.K. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on Β-Alanine and Isatin
Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin by Robert Philip Colaguori A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Pharmaceutical Sciences University of Toronto © Copyright by Robert Philip Colaguori, 2016 ii Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin Robert Philip Colaguori Master of Science Department of Pharmaceutical Sciences University of Toronto 2016 Abstract Epilepsy is the fourth-most common neurological disorder in the world. Approximately 70% of cases can be controlled with therapeutics, however 30% remain pharmacoresistant. There is no cure for the disorder, and patients affected are subsequently medicated for life. Thus, there is a need to develop compounds that can treat not only the symptoms, but also delay/prevent progression. Previous work resulted in the discovery of NC-2505, a substituted β-alanine with activity against chemically induced seizures. Several N- and α-substituted derivatives of this compound were synthesized and evaluated in the kindling model and 4-AP model of epilepsy. In the kindling model, RC1-080 and RC1-102 were able to decrease the mean seizure score from 5 to 3 in aged mice. RC1-085 decreased the interevent interval by a factor of 2 in the 4-AP model. Future studies are focused on the synthesis of further compounds to gain insight on structure necessary for activity. iii Acknowledgments First and foremost, I would like to thank my supervisor Dr. Donald Weaver for allowing me to join the lab as a graduate student and perform the work ultimately resulting in this thesis. -

Staged Anticonvulsant Screening for Chronic Epilepsy
Staged anticonvulsant screening for chronic epilepsy The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Berdichevsky, Yevgeny, Yero Saponjian, Kyung#Il Park, Bonnie Roach, Wendy Pouliot, Kimberly Lu, Waldemar Swiercz, F. Edward Dudek, and Kevin J. Staley. 2016. “Staged anticonvulsant screening for chronic epilepsy.” Annals of Clinical and Translational Neurology 3 (12): 908-923. doi:10.1002/acn3.364. http://dx.doi.org/10.1002/ acn3.364. Published Version doi:10.1002/acn3.364 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:30371039 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA RESEARCH ARTICLE Staged anticonvulsant screening for chronic epilepsy Yevgeny Berdichevsky1,a, Yero Saponjian2,3,a, Kyung-Il Park4,a, Bonnie Roach5, Wendy Pouliot5, Kimberly Lu6, Waldemar Swiercz2,3, F. Edward Dudek5 & Kevin J. Staley2,3 1Department of Electrical and Computer Engineering and Bioengineering Program, Lehigh University, Bethlehem, Pennsylvania 18015 2Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts 02129 3Harvard Medical School, Boston, Massachusetts 02129 4Department of Neurology, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea 06236 5Department of Neurosurgery, University of Utah School of Medicine, Salt Lake City, Utah 84108 6Boston University School of Medicine, Boston, Massachusetts 02119 Correspondence Abstract Kevin J. Staley, Department of Neurology, Massachusetts General Hospital, Boston, MA Objective: Current anticonvulsant screening programs are based on seizures 02129.