Treatment of Myoclonus with Pheneturide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2011/0244057 A1 Ehrenberg (43) Pub
US 2011024.4057A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0244057 A1 Ehrenberg (43) Pub. Date: Oct. 6, 2011 (54) COMBINATION THERAPIES WITH Related U.S. Application Data TOPIRAMATE FOR SEIZURES, RESTLESS LEGS SYNDROME, AND OTHER (60) Provisional application No. 61/100,232, filed on Sep. NEUROLOGICAL CONDITIONS 25, 2008. Publication Classification (51) Int. Cl. (76) Inventor: Bruce L. Ehrenberg, New York, A633/26 (2006.01) NY (US) A63L/35 (2006.01) A633/06 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/120,933 A6IP 25/28 (2006.01) A6IP 25/08 (2006.01) (22) PCT Filed: Sep. 25, 2009 (52) U.S. Cl. .......... 424/648: 514/454; 424/682; 424/646 (57) ABSTRACT (86). PCT No.: PCT/US2009/058495 The invention provides compositions and methods of treating various conditions, including neurological conditions, with S371 (c)(1), pharmaceutical compositions including a sulfamate (e.g., (2), (4) Date: Jun. 9, 2011 topiramate) and magnesium. US 2011/024.4057 A1 Oct. 6, 2011 COMBINATION THERAPES WITH TOPIRAMATE FOR SEIZURES, RESTLESS LEGS SYNDROME, AND OTHER II NEUROLOGICAL CONDITIONS CROSS-REFERENCE TO RELATED APPLICATIONS 0001. This application claims the benefit of the priority date of U.S. Application No. 61/100,232, filed Sep. 25, 2008. For the purpose of any U.S. patent that may issue from the 0005 wherein RandR, are the same or different and are U.S. national phase correlate of the present application, the hydrogen, lower alkyl or are alkyl and are joined to form a content of the priority document is hereby incorporated by cyclopentyl or cyclohexyl ring, and (b) magnesium. -

Staged Anticonvulsant Screening for Chronic Epilepsy
Staged anticonvulsant screening for chronic epilepsy The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Berdichevsky, Yevgeny, Yero Saponjian, Kyung#Il Park, Bonnie Roach, Wendy Pouliot, Kimberly Lu, Waldemar Swiercz, F. Edward Dudek, and Kevin J. Staley. 2016. “Staged anticonvulsant screening for chronic epilepsy.” Annals of Clinical and Translational Neurology 3 (12): 908-923. doi:10.1002/acn3.364. http://dx.doi.org/10.1002/ acn3.364. Published Version doi:10.1002/acn3.364 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:30371039 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA RESEARCH ARTICLE Staged anticonvulsant screening for chronic epilepsy Yevgeny Berdichevsky1,a, Yero Saponjian2,3,a, Kyung-Il Park4,a, Bonnie Roach5, Wendy Pouliot5, Kimberly Lu6, Waldemar Swiercz2,3, F. Edward Dudek5 & Kevin J. Staley2,3 1Department of Electrical and Computer Engineering and Bioengineering Program, Lehigh University, Bethlehem, Pennsylvania 18015 2Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts 02129 3Harvard Medical School, Boston, Massachusetts 02129 4Department of Neurology, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea 06236 5Department of Neurosurgery, University of Utah School of Medicine, Salt Lake City, Utah 84108 6Boston University School of Medicine, Boston, Massachusetts 02119 Correspondence Abstract Kevin J. Staley, Department of Neurology, Massachusetts General Hospital, Boston, MA Objective: Current anticonvulsant screening programs are based on seizures 02129. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

FINAL STUDY PROTOCOL Utilisation of Antiepileptic Medicines in Girls
FINAL STUDY PROTOCOL Utilisation of antiepileptic medicines in girls and women of childbearing potential - a study in three European countries Prepared for the European Medicines Agency May 2017 Version 2.0 Approved 15th May 2017 EUROmediSAFE Consortium 1 TABLE OF CONTENTS Page 1. Background 3 2. Aims 4 3. Data sources 5 4. Methods 6 5. Statistical analyses 12 6. Sample size 15 7. Strengths and limitations 15 8. Study report and manuscript 17 9. Communication of study results 17 10. Ethical and data access approvals 17 11. Milestones 18 12. Quality control 18 13. Data access, storage and sharing 18 14. Protocol authors 20 15. Amendments and deviations from the protocol 20 Appendix I 21 2 1. BACKGROUND In October 2013, the Medicines and Healthcare Regulatory Authority issued a referral into the use of sodium valproate in girls and women of childbearing potential, following new evidence in the literature relating to an increased risk of neurodevelopmental disorders in children exposed to sodium valproate in-utero. The review was carried out by the Pharmacovigilance Risk Assessment Committee (PRAC) and in October 2014 the PRAC adopted its recommendation. Following completion of the review, a letter was sent to healthcare professionals in January 2015 informing them of the changes in the recommendations for valproate prescribing. The recommendations resulting from the review included that Valproate and related substances should not be used in female children, women of childbearing potential and pregnant women unless alternative treatments are ineffective or not tolerated. Valproate and related substances should be contraindicated in prophylaxis of migraine attacks in pregnancy and women of childbearing potential who are not using effective methods of contraception during treatment with valproate. -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

Catalyst Rx 2010 National Preferred Formulary
Vantage Health Plan Formulary VHP674 Current as of December 31, 2012 2013 Vantage Catamaran Advantage Formulary Benefit designs may vary and formulary changes can occur at any time Effective January 1, 2013 Introduction The Vantage Catamaran Advantage Formulary was developed to serve as a guide for physicians, pharmacists, health care professionals and members in the selection of cost-effective drug therapy. Catamaran recognizes that drug therapy is an integral part of effective health management. The vast availability of medication options, however, warrants a reasonable program for drug selection and use. Catamaran continually reviews new and existing medications to ensure the Formulary remains responsive to the needs of our members and health professionals. Criteria used to evaluate drug selection for the formulary includes, but is not limited, to: safety, efficacy and cost-effectiveness data, as well as comparison of relevant benefits of similar prescription or over-the counter (OTC) agents while minimizing potential duplications. The formulary is a continually reviewed and modified document that represents the prevailing clinical opinion of Catamaran and Vantage Health Plan. This dynamic process does not allow this document to be completely accurate at all times. To accommodate regular changes, an updated electronic version of this formulary is available online at www.vhpla.com. Vantage and Catamaran welcomes your input and feedback on the information provided in this document. How to Read the Formulary All drugs are listed by their generic names and most common proprietary (branded) name. Specific drug listings may be accessed by using the index, either by generic (in lowercase) or proprietary name (in uppercase) and by therapeutic drug category. -
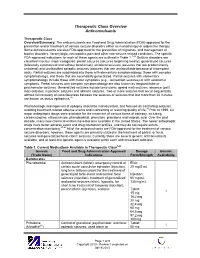
Therapeutic Class Overview Anticonvulsants
Therapeutic Class Overview Anticonvulsants Therapeutic Class Overview/Summary: The anticonvulsants are Food and Drug Administration (FDA)-approved for the prevention and/or treatment of various seizure disorders either as monotherapy or adjunctive therapy. Some anticonvulsants are also FDA-approved for the prevention of migraines, and management of bipolar disorders, fibromyalgia, neuropathic pain and other non-seizure related conditions. The specific FDA-approved indications for each of these agents are outlined in Table 1.1-44 Seizure disorders are classified into four major categories: partial seizures (seizures beginning locally), generalized seizures (bilaterally symmetrical and without local onset), unilateral seizures (seizures that are predominantly unilateral) and unclassified epileptic seizures (seizures that are unclassifiable because of incomplete data). Partial seizures are subdivided into those with elementary symptomatology, those with complex symptomatology, and those that are secondarily generalized. Partial seizures with elementary symptomatology include those with motor symptoms (e.g., Jacksonian seizures) or with autonomic symptoms. Partial seizures with complex symptomatology are also known as temporal lobe or psychomotor seizures. Generalized seizures include tonic-clonic (grand mal) seizures, absence (petit mal) seizures, myoclonic seizures and akinetic seizures. Two or more seizures that occur sequentially without full recovery of consciousness between the seizures or seizures that last more than 30 minutes are known as status epilepticus.45 Pharmacologic management of epilepsy should be individualized, and focused on controlling seizures, avoiding treatment-related adverse events and maintaining or restoring quality of life.46 Prior to 1990, six major antiepileptic drugs were available for the treatment of various forms of epilepsy, including carbamazepine, ethosuximide, phenobarbital, phenytoin, primidone and valproic acid. -

Showed Atrophy and Cystic Changes in the Left Hemisphere with Compensatory Dilatation of the Lateral Ventricle
increased. MRI showed atrophy and cystic changes in the left hemisphere with compensatory dilatation of the lateral ventricle. Thalidomide (300 mg/day) was added to a midazolam iv drip, and partial seizure control was sustained. For the past 3 years, her seizures have been less intense and less frequent and have not interfered with everyday activities. Treatment includes thalidomide 5 mg/kg, valproate 30 mg/kg, clonazepam and piracetam. Apart from occasional leukopenia, no other adverse effects are reported. (Maijanovic BD, Stojanov LM, Zdravkovic DS et al. Rasmusen syndrome and long-term response to thalidomide. Pediatr Neurol Aug 2003;29:151-156). (Respond: Dr Maijanovic, Department of Neurology, Pediatric Clinic of Mother and Child Health Care Institute, Belgrade, Yugoslavia). COMMENT. Thalidomide may be considered as an alternative treatment for refractory and incapacitating seizures in Rasmussen syndrome, when more conventional therapies have failed. One previous report found thalidomide effective in a 7-year-old male with Rasmussen syndrome whose seizures were associated with high levels of CSF tumor necrosis factor a. (Ravenscroft A et al. Brain Dev 1998;20:398). The antiepileptic effect may be related to inhibition of the tumor necrosis factor and boosting of the immune response. Apart from the high teratogenicity, a sensory neuropathy is the major adverse complication of thalidomide therapy. LEVETIRACETAM IN LANDAU-KLEFFNER SYNDROME A 5-year-old girl with seizures and progressive language deterioration and a diagnosis of Landau-Kleffner syndrome was benefited by treatment with levetiracetam at Johns Hopkins Hospital, Baltimore, MD. Video-EEG monitoring showed continuous 2- to 3-Hz spike-wave discharges, maximal left, during sleep. -

The Risk of Specific Congenital Anomalies in Relation to Newer Antiepileptic Drugs
Drugs - Real World Outcomes (2016) 3:131–143 DOI 10.1007/s40801-016-0078-1 REVIEW ARTICLE The Risk of Specific Congenital Anomalies in Relation to Newer Antiepileptic Drugs: A Literature Review 1 2 1 Josta de Jong • Ester Garne • Lolkje T. W. de Jong-van den Berg • Hao Wang1 Published online: 24 May 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com Abstract found. The signals for associations between topiramate and Background More information is needed about possible cleft lip with/without cleft palate and hypospadias were associations between the newer anti-epileptic drugs considered strong. Associations between lamotrigine and (AEDs) in the first trimester of pregnancy and specific anencephaly and transposition of great vessels were found congenital anomalies of the fetus. within one study and were not supported by other studies. Objectives We performed a literature review to find sig- No signals were found for the other newer AEDs, or the nals for potential associations between newer AEDs information was too limited to provide such a signal. (lamotrigine, topiramate, levetiracetam, gabapentin, Conclusion In terms of associations between monotherapy oxcarbazepine, eslicarbazepine, felbamate, lacosamide, with a newer AED in the first trimester of pregnancy and a pregabalin, retigabine, rufinamide, stiripentol, tiagabine, specific congenital anomaly, the signals for topiramate and vigabatrin, and zonisamide) and specific congenital cleft lip with/without cleft palate and hypospadias should anomalies. be investigated further. Methods We searched PubMed and EMBASE to find observational studies with pregnancies exposed to newer AEDs and detailed information on congenital anomalies. Key Points The congenital anomalies in the studies were classified according to the congenital anomaly subgroups of Euro- Information was found on specific congenital pean Surveillance of Congenital Anomalies (EUROCAT). -

Antiepileptic Drugs: Evolution of Our Knowledge and Changes in Drug Trials
ILAE 110th anniversary review paper* Epileptic Disord 2019; 21 (4): 319-29 Antiepileptic drugs: evolution of our knowledge and changes in drug trials Emilio Perucca Past President of the International League Against Epilepsy Division of Clinical and Experimental Pharmacology, Department of Internal Medicine and Therapeutics, University of Pavia, Pavia and IRCCS Mondino Foundation, Pavia, Italy Received April 30, 2019; Accepted June 01, 2019 ABSTRACT – Clinical trials provide the evidence needed for rational use of medicines. The evolution of drug trials follows largely the evolution of regulatory requirements. This article summarizes methodological changes in antiepileptic drug trials and associated advances in knowledge starting from 1938, the year phenytoin was introduced and also the year when evi- dence of safety was made a requirement for the marketing of medicines in the United States. The first period (1938-1969) saw the introduction of over 20 new drugs for epilepsy, many of which did not withstand the test of time. Only few well controlled trials were completed in that period and trial designs were generally suboptimal due to methodological constraints. The intermediate period (1970-1988) did not see the introduction of any major new medication, but important therapeutic advances took place due to improved understanding of the properties of available drugs. The value of therapeutic drug monitoring and monotherapy were recognized dur- ing the intermediate period, which also saw major improvements in trial methodology. The last period (1989-2019) was dominated by the introduc- tion of second-generation drugs, and further evolution in the design of monotherapy and adjunctive-therapy trials. The expansion of the pharma- cological armamentarium has improved opportunities for tailoring drug treatment to the characteristics of the individual. -

Folavit 5 Mg Tablets
1. NAME OF THE MEDICINAL PRODUCT Folavit 1 mg tablets Folavit 5 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Folavit 1 mg tablets Each tablet contains 1 mg folic acid. Excipients with known effect: each tablet contains 72,2 mg lactose (as monohydrate) and 8,3 mg sucrose. For the full list of excipients, see section 6.1. Folavit 5 mg tablets Each tablet contains 5 mg folic acid. Excipients with known effect: each tablet contains 68,4 mg lactose (as monohydrate) and 8,3 mg sucrose. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet Folavit 1 mg tablets Light yellow or light yellow orange speckled, round, biconvex tablet of 7 mm with a score-line on one side. The tablet can be divided into two equal doses. Folavit 5 mg tablets Yellow or yellow orange speckled, round, biconvex tablet of 7 mm with a score-line on one side. The tablet can be divided into two equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Folavit is indicated in adults and children. - Reduction of side effects in patients receiving low-dose methotrexate therapy for rheumatoid arthritis, psoriasis and inflammatory bowel disease. - Prevention or treatment of folate deficiency, which may cause macrocytic anemia. Folate deficiency is associated with elevated homocysteine levels. Various conditions can cause folate deficiency: - Inadequate dietary intake (e.g. malnutrition in the elderly) - Malabsorption (e.g. coeliac disease, bariatric surgery, inflammatory bowel disease, alcoholism) - Increased utilization (e.g. pregnancy, lactation, hemolytic anemia) - Increased loss (e.g. hemodialysis) 1 - Intake of medications (e.g. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0