SUPPLEMENT Etable 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Drug-Induced Angle-Closure Glaucoma
10.5005/jp-journals-10008-1100 ArujREVIEW K Khurana ARTICLE et al Drug-induced Angle-Closure Glaucoma Aruj K Khurana, Bhawna Khurana, Ashok K Khurana Regional Institute of Ophthalmology, Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India Correspondence: Ashok K Khurana, Senior Professor, Regional Institute of Ophthalmology, Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India ABSTRACT Drug-induced angle-closure glaucoma is an important entity for the ophthalmologist as well as the general physician as it represents a preventable cause of potential blindness. This brief review highlights the fact that a high index of suspicion, in a susceptible individual followed by confirmation on appropriate imaging modality (UBM, ultrasound or anterior segment OCT) can alleviate the threat to sight and also help to institute appropriate therapy. Keywords: Acute angle closure, Drug-induced glaucoma. INTRODUCTION factors exist for this syndrome.9 Other sulfa-based drugs known Drug-induced angle-closure glaucoma is an important entity to be associated with AACG: Acetazolamide, hydrochloro- 10 for the ophthalmologist as well as the general physician as it thiazide and cotrimoxazole. represents a preventable cause of potential blindness.1 Acute Anticholinergic drugs implicated in the causation of AACG angle-closure glaucoma can develop in a susceptible individual include atropine, homatropine, cyclopentolate and ipratropium by various classes of drugs.2 Practitioners using any of these bromide.10,11 Atropine is often used to treat bradycardia, drugs should be aware of their potential to cause acute angle especially related to general anesthesia. Postoperative AACG closure, such that a patient presenting with signs or symptoms has been reported in patients after general anesthesia for of acute angle closure should be immediately referred to an abdominal, orthopedic, facial and endoscopic surgery.12 3 ophthalmologist. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0244057 A1 Ehrenberg (43) Pub
US 2011024.4057A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0244057 A1 Ehrenberg (43) Pub. Date: Oct. 6, 2011 (54) COMBINATION THERAPIES WITH Related U.S. Application Data TOPIRAMATE FOR SEIZURES, RESTLESS LEGS SYNDROME, AND OTHER (60) Provisional application No. 61/100,232, filed on Sep. NEUROLOGICAL CONDITIONS 25, 2008. Publication Classification (51) Int. Cl. (76) Inventor: Bruce L. Ehrenberg, New York, A633/26 (2006.01) NY (US) A63L/35 (2006.01) A633/06 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/120,933 A6IP 25/28 (2006.01) A6IP 25/08 (2006.01) (22) PCT Filed: Sep. 25, 2009 (52) U.S. Cl. .......... 424/648: 514/454; 424/682; 424/646 (57) ABSTRACT (86). PCT No.: PCT/US2009/058495 The invention provides compositions and methods of treating various conditions, including neurological conditions, with S371 (c)(1), pharmaceutical compositions including a sulfamate (e.g., (2), (4) Date: Jun. 9, 2011 topiramate) and magnesium. US 2011/024.4057 A1 Oct. 6, 2011 COMBINATION THERAPES WITH TOPIRAMATE FOR SEIZURES, RESTLESS LEGS SYNDROME, AND OTHER II NEUROLOGICAL CONDITIONS CROSS-REFERENCE TO RELATED APPLICATIONS 0001. This application claims the benefit of the priority date of U.S. Application No. 61/100,232, filed Sep. 25, 2008. For the purpose of any U.S. patent that may issue from the 0005 wherein RandR, are the same or different and are U.S. national phase correlate of the present application, the hydrogen, lower alkyl or are alkyl and are joined to form a content of the priority document is hereby incorporated by cyclopentyl or cyclohexyl ring, and (b) magnesium. -
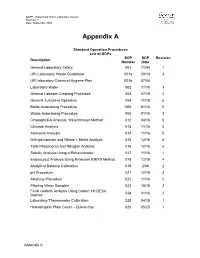
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

Staged Anticonvulsant Screening for Chronic Epilepsy
Staged anticonvulsant screening for chronic epilepsy The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Berdichevsky, Yevgeny, Yero Saponjian, Kyung#Il Park, Bonnie Roach, Wendy Pouliot, Kimberly Lu, Waldemar Swiercz, F. Edward Dudek, and Kevin J. Staley. 2016. “Staged anticonvulsant screening for chronic epilepsy.” Annals of Clinical and Translational Neurology 3 (12): 908-923. doi:10.1002/acn3.364. http://dx.doi.org/10.1002/ acn3.364. Published Version doi:10.1002/acn3.364 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:30371039 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA RESEARCH ARTICLE Staged anticonvulsant screening for chronic epilepsy Yevgeny Berdichevsky1,a, Yero Saponjian2,3,a, Kyung-Il Park4,a, Bonnie Roach5, Wendy Pouliot5, Kimberly Lu6, Waldemar Swiercz2,3, F. Edward Dudek5 & Kevin J. Staley2,3 1Department of Electrical and Computer Engineering and Bioengineering Program, Lehigh University, Bethlehem, Pennsylvania 18015 2Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts 02129 3Harvard Medical School, Boston, Massachusetts 02129 4Department of Neurology, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea 06236 5Department of Neurosurgery, University of Utah School of Medicine, Salt Lake City, Utah 84108 6Boston University School of Medicine, Boston, Massachusetts 02119 Correspondence Abstract Kevin J. Staley, Department of Neurology, Massachusetts General Hospital, Boston, MA Objective: Current anticonvulsant screening programs are based on seizures 02129. -

Anticholinergic Drugs and Dementia
DEMENTIA Q&A 24 Anticholinergic drugs and dementia Anticholinergics are a class of drug used to treat a wide variety of medical conditions. As with any medication, anticholinergics can have many beneficial effects, but these need to be balanced against a number of potential risks. This sheet provides information about how these drugs work as well as the impact that they may have in respect to dementia risk and cognitive functioning. What are anticholinergics? in time.4 It has been estimated that in Australia, 33% of people over the age of 65 years take enough Anticholinergics are generally used to inhibit the medications with anticholinergic effects to potentially involuntary movements of muscles or balance the increase their risk of harm.5 The use of anticholinergics production of various chemicals within the body. They is more common in older people because these drugs have been used in treating a wide variety of medical are prescribed for the symptomatic management of conditions including symptoms of psychosis (which medical conditions that often occur in later life. can be caused by bipolar disorder and schizophrenia), depression, urinary incontinence (e.g. overactive How do anticholinergic drugs work? bladder), gastrointestinal spasms, allergies, respiratory Anticholinergics are a class of drug that block the conditions (such as asthma and chronic obstructive action of acetylcholine in the nervous system, a pulmonary disease), Parkinson’s disease, conditions chemical (neurotransmitter) that is used to control concerning muscles in the eye and pupil dilation, messages travelling from one cell to another. They do nausea, sleep disorders and cardiovascular complaints this by blocking the binding of acetylcholine to its (slow heart rhythms) (Table 1).1,2 Some cold and flu receptor in the nerve cells. -

Pharmacology of Ophthalmic Agents
Ophthalmic Pharmacology Richard Alan Lewis M.D., M.S., PHARMACOLOGY FOPS PHARMACOKINETICS OF Professor, Departments of Ophthalmology, • The study of the absorption, OPHTHALMIC Medicine, Pediatrics, and Molecular distribution, metabolism, AGENTS and Human Genetics and excretion of a drug or and the National School of Tropical agent Introduction and Review Medicine Houston, Texas PHARMACOKINETICS Factors Affecting Drug Penetration Factors Affecting Drug Penetration into Ocular Tissues • A drug can be delivered to ocular tissue: into Ocular Tissues – Locally: • Drug concentration and solubility: The higher the concentration the better the penetration, • Surfactants: The preservatives in ocular • Eye drop but limited by reflex tearing. preparations alter cell membrane in the cornea • Ointment and increase drug permeability, e.g., • Viscosity: Addition of methylcellulose and benzalkonium and thiomersal • Periocular injection polyvinyl alcohol increases drug penetration by • pH: The normal tear pH is 7.4; if the drug pH is • Intraocular injection increasing the contact time with the cornea and altering corneal epithelium. much different, it will cause reflex tearing. – Systemically: • Lipid solubility: Because of the lipid rich • Drug tonicity: When an alkaloid drug is put in • Orally environment of the epithelial cell membranes, relatively alkaloid medium, the proportion of the uncharged form will increase, thus more • IM the higher lipid solubility, the more the penetration. • IV penetration. FLUORESCEIN FLUORESCEIN Chemistry Dosage ● C20H1205, brown crystal ● Adults: 500-750 mg IV ● M.W. 322.3 e.g., 3 cc 25% solution ● Peak absorption 485-500 nm. 5 cc 10% solution ● Peak emission 520-530 nm. ● Children: 1.5-2.5 mg/kg IV Richard Alan Lewis, M.D., M.S.