Tegretol (Carbamazepine)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
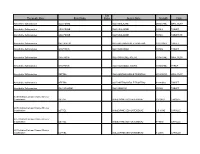
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Is Alcohol Use Related to High Cholesterol in Premenopausal
Research iMedPub Journals Journal of Preventive Medicine 2018 http://www.imedpub.com/ Vol.3 No.1:3 ISSN 2572-5483 DOI: 10.21767/2572-5483.100024 Is Alcohol Use Related To High Cholesterol in Premenopausal Women Aged 40-51 Years Old? Sydnee Homeyer, Jessica L Hartos*, Holli Lueg, Jessica Moore and Patricia Stafford Department of Physician Assistant Studies, University of North Texas Health Science Center, USA *Corresponding author: Jessica L. Hartos, Department of Physician Assistant Studies, University of North Texas Health Science Center, USA, Tel: 817-735-2454; Fax: 817-735-2529; E-mail: [email protected] Received date: November 13, 2017; Accepted date: January 8, 2018; Publication date: January 15, 2018 Citation: Homeyer S, Hartos JL, Lueg H, Moore J, Stafford P, et al. (2018) Is Alcohol Use Related To High Cholesterol In Premenopausal Women Aged 40-51 Years Old? J Prev Med Vol.3 No.3: 3. Copyright: © 2018 Homeyer S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Introduction Abstract Increased serum cholesterol levels is a major risk factor for coronary heart disease (CHD) [1], and over seventy-one million Purpose: Alcohol use and cholesterol are related in men and American adults have high cholesterol (27%) [2,3]. In American postmenopausal women but relations between alcohol use women, cholesterol levels have been shown to increase with and cholesterol are unclear for premenopausal women. The age, and nearly 1 in 2 American women has high or borderline purpose of this study was to determine whether alcohol use was related to cholesterol in women aged 40-51 years old. -

E the Effect of Topiramate on Weight Loss in Patients with Type 2 Diabetes
E The effect of topiramate on weight loss in patients with type 2 diabetes RTICL Sedighe Moradi, Scott Reza Jafarian Kerman, Mina Mollabashi A Institute of Endocrinology and Metabolism (Hemmat Campus), Firoozgar Hospital, Tehran University of Medical Sciences, Tehran, Iran Background: Obesity has been associated with several co‑morbidities such as diabetes and increased mortality. In general, the use of medication promotes only a modest weight loss in the range of 2 to 10 kg, usually most effective during the first 6 months of therapy; however, studies have shown positive effects on other risk factors such as blood pressure and serum glucose levels, but there are fewer studies in patients with diabetes. The aim of this study was to assess the effect of topiramate on weight reduction patients with type 2 diabetes. Materials and Methods: This was a 32‑week randomized clinical trial study of 69 subjects during 2008‑2010. RIGINAL Patients, in two treatment groups were given topiramate (39 patients) and Placebo (30 patients) and were subjected to participation in a non‑pharmacologic lifestyle intervention program; which were randomly allocated in our two groups. The percentage change in body O weight and Body Mass Index (BMI) at the end of the study was the primary efficacy endpoint and secondary indicators were changes in blood pressure (BP), proportion of subjects who achieved 5% or 10% weight loss, changes in lipid profile (total cholesterol, low‑density lipoprotein cholesterol, high‑density lipoprotein cholesterol, triglycerides); and changes in glycosylated hemoglobin (HgA1c). Paired samples and independent samples t‑test was used for statistical analysis. -

(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

Clinical Review, Adverse Events
Clinical Review, Adverse Events Drug: Carbamazepine NDA: 16-608, Tegretol 20-712, Carbatrol 21-710, Equetro Adverse Event: Stevens-Johnson Syndrome Reviewer: Ronald Farkas, MD, PhD Medical Reviewer, DNP, ODE I 1. Executive Summary 1.1 Background Carbamazepine (CBZ) is an anticonvulsant with FDA-approved indications in epilepsy, bipolar disorder and neuropathic pain. CBZ is associated with Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), closely related serious cutaneous adverse drug reactions that can be permanently disabling or fatal. Other anticonvulsants, including phenytoin, phenobarbital, and lamotrigine are also associated with SJS/TEN, as are members of a variety of other drug classes, including nonsteriodal anti-inflammatory drugs and sulfa drugs. The incidence of CBZ-associated SJS/TEN has been considered “extremely rare,” as noted in current U.S. drug labeling. However, recent publications and postmarketing data suggest that CBZ- associated SJS/TEN occurs at a much higher rate in some Asian populations, about 2.5 cases per 1,000 new exposures, and that most of this increased risk is in individuals carrying a specific human leukocyte antigen (HLA) allele, HLA-B*1502. This HLA-B allele is present in about 5- to 20% of many, but not all, Asian populations, and is also present in about 2- to 4% of South Asians/Indians. The allele is also present at a lower frequency, < 1%, in several other ethnic groups around the world (although likely due to distant Asian ancestry). About 10% of U.S. Asians carry HLA-B*1502. HLA-B*1502 is generally not present in the U.S. -

Diazepam and Kava Combination Article
Journal of Advanced Research (2014) 5, 587–594 Cairo University Journal of Advanced Research ORIGINAL ARTICLE Enhanced efficacy and reduced side effects of diazepam by kava combination Rasha A. Tawfiq a, Noha N. Nassar b,*, Wafaa I. El-Eraky c, Ezzeldein S. El-Denshary b a Egyptian Patent Office, Academy of Scientific Research and Technology, 101 Kasr El-Eini St., Cairo, Egypt b Department of Pharmacology and Toxicology, Faculty of Pharmacy, Cairo University, Kasr El-Eini St., Cairo, Egypt c Department of Pharmacology, National Research Center, El-Tahrir St., Giza, Egypt ARTICLE INFO ABSTRACT Article history: The long term use of antiepileptic drugs possesses many unwanted effects; thus, new safe com- Received 2 April 2013 binations are urgently mandated. Hence, the present study aimed to investigate the anticonvul- Received in revised form 18 July 2013 sant effect of kava alone or in combination with a synthetic anticonvulsant drug, diazepam Accepted 15 August 2013 (DZ). To this end, female Wistar rats were divided into two subsets, each comprising 6 groups Available online 22 August 2013 as follows: group (i) received 1% Tween 80 p.o. and served as control, while groups (ii) and (iii) received kava at two dose levels (100 and 200 mg/kg, p.o.). The remaining three groups received Keywords: (iv) DZ alone (10 mg/kg p.o.) or kava in combination with DZ (v) (5 mg/kg, p.o.) or (vi) (10 mg/ Kava kg, p.o.). Results of the present study revealed that kava increased the maximal electroshock Diazepam seizure threshold (MEST) and enhanced the anticonvulsant effect of diazepam following both Anticonvulsant acute and chronic treatment. -

Kava Kava Extract Is Available from Ashland Chemical Co., Mini Star International, Inc., and QBI (Quality Botanical Ingredients, Inc.)
SUMMARY OF DATA FOR CHEMICAL SELECTION Kava Kava 9000-38-8; 84696-40-2 November 1998 TABLE OF CONTENTS Basis for Nomination Chemical Identification Production Information Use Pattern Human Exposure Regulatory Status Evidence for Possible Carcinogenic Activity Human Data Animal Data Metabolism Other Biological Effects Structure-Activity Relationships References BASIS OF NOMINATION TO THE CSWG Kava kava is brought to the attention of the CSWG because it is a rapidly growing, highly used dietary supplement introduced into the mainstream U.S. market relatively recently. Through this use, millions of consumers using antianxiety preparations are potentially exposed to kava kava. A traditional beverage of various Pacific Basin countries, kava clearly has psychoactive properties. The effects of its long-term consumption have not been documented adequately; preliminary studies suggest possibly serious organ system effects. The potential carcinogenicity of kava and its principal constituents are unknown. INPUT FROM GOVERNMENT AGENCIES/INDUSTRY The U.S. Pharmacopeia is in the process of reviewing kava kava. No decision on preparation of a monograph has been made. SELECTION STATUS ACTION BY CSWG: 12/14/98 Studies requested: - Toxicological evaluation, to include studies of reproductive toxicity and neurotoxicity - Genotoxicity Priority: High Rationale/Remarks: - Significant human exposure - Leading dietary supplement with rapidly growing use - Concern that kava has been promoted as a substitute for ritilin in children - Test extract standardized to 30 percent kavalactones - NCI is conducting studies in Salmonella typhimurium CHEMICAL IDENTIFICATION CAS Registry Number: 9000-38-8 Kava-kava resin (8CI) Chemical Abstract Service Name: 84696-40-2 CAS Registry Number: Pepper (Piper), P. methysticum, ext. Chemical Abstract Service Name: Extract of kava; kava extract; Piper Synonyms and Trade Names: methisticum extract Description: The tropical shrub Piper methysticum is widely cultivated in the South Pacific. -

Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence
International Journal of Molecular Sciences Review Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence Giulia Maria Camerino 1, Nancy Tarantino 1 , Ileana Canfora 1 , Michela De Bellis 1, Olimpia Musumeci 2 and Sabata Pierno 1,* 1 Section of Pharmacology, Department of Pharmacy and Drug Sciences, University of Bari “Aldo Moro”, 70125 Bari, Italy; [email protected] (G.M.C.); [email protected] (N.T.); [email protected] (I.C.); [email protected] (M.D.B.) 2 Unit of Neurology and Neuromuscular Disorders, Department of Clinical and Experimental Medicine, University of Messina, 98122 Messina, Italy; [email protected] * Correspondence: [email protected] Abstract: Statins are the most prescribed and effective drugs to treat cardiovascular diseases (CVD). Nevertheless, these drugs can be responsible for skeletal muscle toxicity which leads to reduced compliance. The discontinuation of therapy increases the incidence of CVD. Thus, it is essential to assess the risk. In fact, many studies have been performed at preclinical and clinical level to investigate pathophysiological mechanisms and clinical implications of statin myotoxicity. Conse- quently, new toxicological aspects and new biomarkers have arisen. Indeed, these drugs may affect gene transcription and ion transport and contribute to muscle function impairment. Identifying a marker of toxicity is important to prevent or to cure statin induced myopathy while assuring the right therapy for hypercholesterolemia and counteracting CVD. In this review we focused on the mechanisms of muscle damage discovered in preclinical and clinical studies and highlighted the Citation: Camerino, G.M.; Tarantino, pathological situations in which statin therapy should be avoided. -

Alcohol and the Cardiovascular System
Alcohol and the Cardiovascular System Molecular Mechanisms for Beneficial and Harmful Action SAM ZAKHARI, PH.D. Alcohol can be beneficial or harmful to the cardiovascular system, depending on the amount consumed and the characteristics of the consumer. Of the numerous cellular and molecular mechanisms that are thought to explain the beneficial effects of moderate drinking, this article discusses four, involving (1) high density lipoproteins, (2) cellular signaling, (3) platelet function in blood clot formation, and (4) stimulation of blood clot dissolution. Although light-to-moderate drinking can protect against coronary artery disease, heavy alcohol consumption can damage the cardiovascular system, resulting in maladies such as heart muscle disorders, irregular heart rhythms, high blood pressure, and strokes. This article summarizes representative epidemiological and animal studies on these cardiovascular consequences of chronic heavy alcohol consumption and reviews mechanisms that have been suggested to explain alcohol’s effects. KEY WORDS: chronic AODE (alcohol and other drug effects); molecular interaction; biochemical mechanism; lipoproteins; platelets; blood coagulation; moderate AOD use; heavy AOD use; therapeutic drug effect; AODR (alcohol and other drug related) disorder; alcoholic cardiomyopathy; cardiac arrhythmia; hypertensive disorder; stroke; literature review; cell signaling lcoholic beverages have been sumed. This chronic heavy drinking1 is used—and abused—since the 1The term “heavy drinking” is not used consistently in a significant factor in the development the alcohol literature; therefore, this article generally dawn of history. Although most 2 A of alcohol dependence, or alcoholism, refers to “heavy drinking” and “heavy drinkers” based people who choose to drink can limit their and is associated with serious adverse on the terms used in the reference cited. -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine