Antimicrobial & Anticonvulsant Activity Show Some Newer Phthalimide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
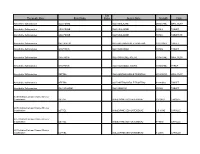
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Hydantoin and Succinimide-Substituted
(1 9) J Eur°Pean Patent Office <*S Office europeen des brevets (11) EP 0 533 243 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) int. CI.6: C07D 401/1 2, C07D 471/10, of the grant of the patent: A61 K 31/445 Q07D 401 /1 4 17,2,997 Buiietin 1997/51 // (C07D471/1 0, 235:00, (21) Application number: 92202723.0 221:00) (22) Date of filing: 08.09.1992 (54) Hydantoin and succinimide-substituted spiroindanylcamphorsulfonyl derivatives Hydantoin und Succinimid-substituierte Spiroindanylcamphorsulfonyl als Oxytocin-Antagonisten Derives de spiroindanylcamphorsulfonyl substitues par un radical hydantoine ou succinimide comme antagonists d'oxytocine (84) Designated Contracting States: (74) Representative: CH DE FR GB IT LI NL Thompson, John Dr. et al Merck & Co., Inc. (30) Priority: 16.09.1991 US 760416 European Patent Department Terlings Park (43) Date of publication of application: Eastwick Road 24.03.1993 Bulletin 1993/12 Harlow, Essex CM20 2QR (GB) (73) Proprietor: Merck & Co., Inc. (56) References cited: Rahway New Jersey 07065-0900 (US) EP-A- 0 444 945 EP-A- 0 445 974 EP-A- 0 450 761 EP-A- 0 486 280 (72) Inventors: US-A- 3 654 287 • Gilbert, Kevin Bally, PA 19503 (US) • EP-A- 0 444 945 • Williams, Peter D. • EP-A- 0 445 974 Harleysville, PA 19438 (US) • EP-A- 0 450 761 • Hobbs, Doug W. • EP-A- 0 486 280 Lansdale, PA 19446 (US) • US-A- 3 654 287 • Evans, Ben E. Lansdale, PA 19446 (US) Remarks: • Veber, Daniel F. The file contains technical information submitted Ambler, PA 19002 (US) after the application was filed and not included in this specification CO CO CM CO CO Note: Within nine months from the publication of the mention of the grant of the European patent, give IO any person may notice to the European Patent Office of opposition to the European patent granted. -
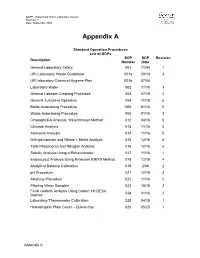
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Pharmacokinetic Drug–Drug Interactions Among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients
International Journal of Molecular Sciences Review Pharmacokinetic Drug–Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients Marta Kara´zniewicz-Łada 1 , Anna K. Główka 2 , Aniceta A. Mikulska 1 and Franciszek K. Główka 1,* 1 Department of Physical Pharmacy and Pharmacokinetics, Poznan University of Medical Sciences, 60-781 Pozna´n,Poland; [email protected] (M.K.-Ł.); [email protected] (A.A.M.) 2 Department of Bromatology, Poznan University of Medical Sciences, 60-354 Pozna´n,Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-(0)61-854-64-37 Abstract: Anti-epileptic drugs (AEDs) are an important group of drugs of several generations, rang- ing from the oldest phenobarbital (1912) to the most recent cenobamate (2019). Cannabidiol (CBD) is increasingly used to treat epilepsy. The outbreak of the SARS-CoV-2 pandemic in 2019 created new challenges in the effective treatment of epilepsy in COVID-19 patients. The purpose of this review is to present data from the last few years on drug–drug interactions among of AEDs, as well as AEDs with other drugs, nutrients and food. Literature data was collected mainly in PubMed, as well as google base. The most important pharmacokinetic parameters of the chosen 29 AEDs, mechanism of action and clinical application, as well as their biotransformation, are presented. We pay a special attention to the new potential interactions of the applied first-generation AEDs (carba- Citation: Kara´zniewicz-Łada,M.; mazepine, oxcarbazepine, phenytoin, phenobarbital and primidone), on decreased concentration Główka, A.K.; Mikulska, A.A.; of some medications (atazanavir and remdesivir), or their compositions (darunavir/cobicistat and Główka, F.K. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Calcium Channel Blocker As a Drug Candidate for the Treatment of Generalised Epilepsies
UNIVERSITAT DE BARCELONA Faculty of Pharmacy and Food Sciences Calcium channel blocker as a drug candidate for the treatment of generalised epilepsies Final degree project Author: Janire Sanz Sevilla Bachelor's degree in Pharmacy Primary field: Organic Chemistry, Pharmacology and Therapeutics Secondary field: Physiology, Pathophysiology and Molecular Biology March 2019 This work is licensed under a Creative Commons license ABBREVIATIONS AED antiepileptic drug AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ANNA-1 antineuronal nuclear antibody 1 BBB blood-brain barrier Bn benzyl BnBr benzyl bromide BnNCO benzyl isocyanate Boc tert-butoxycarbonyl Bu4NBr tetrabutylammonium bromide Ca+2 calcium ion CACNA1 calcium channel voltage-dependent gene cAMP cyclic adenosine monophosphate CCB calcium channel blocker cGMP cyclic guanosine monophosphate CH3CN acetonitrile Cl- chlorine ion Cmax maximum concentration CMV cytomegalovirus CTScan computed axial tomography DCM dichloromethane DIPEA N,N-diisopropylethylamine DMF dimethylformamide DMPK drug metabolism and pharmacokinetics DNET dysembryoplastic neuroepithelial tumours EEG electroencephalogram EPSP excitatory post-synaptic potential FDA food and drug administration Fe iron FLIPR fluorescence imaging plate reader fMRI functional magnetic resonance imaging GABA γ-amino-α-hydroxybutyric acid GAD65 glutamic acid decarboxylase 65 GAERS generalised absence epilepsy rat of Strasbourg GluR5 kainate receptor GTC generalised tonic-clonic H+ hydrogen ion H2 hydrogen H2O dihydrogen dioxide (water) -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Clonazepam/Ethosuximide 479 Abnormal Movements, and for the Treatment of Panic BNFC Suggests Giving the Following Doses by Slow Intravenous 3
Clonazepam/Ethosuximide 479 abnormal movements, and for the treatment of panic BNFC suggests giving the following doses by slow intravenous 3. Peled R, Lavie P. Double-blind evaluation of clonazepam on pe- injection over at least 2 minutes according to age: riodic leg movements in sleep. J Neurol Neurosurg Psychiatry disorder (see Psychiatric Disorders, below). 1987; 50: 1679–81. For epilepsy and myoclonus treatment is started with • neonates: 100 micrograms/kg, repeated if necessary after 24 4. Saletu M, et al. Restless legs syndrome (RLS) and periodic limb hours movement disorder (PLMD): acute placebo-controlled sleep lab- small doses that are progressively increased to an opti- oratory studies with clonazepam. Eur Neuropsychopharmacol • 1 month to 12 years: 50 micrograms/kg (maximum 1 mg), re- 2001; 11: 153–61. mum dose according to response. Total daily doses peated if necessary may initially be taken in 3 or 4 divided doses; however, 5. Saletu A, et al. On the pharmacotherapy of sleep bruxism: place- Older children may be given the usual adult dose. bo-controlled polysomnographic and psychometric studies with once the maintenance dose has been reached, the daily clonazepam. Neuropsychobiology 2005; 51: 214–25. In children aged over 1 month, these doses by injection may be amount may be given as a single dose at night. In the followed by an intravenous infusion of 10 micrograms/kg per Stiff-man syndrome. Clonazepam has been used as an alter- UK the initial oral dose is 1 mg (500 micrograms in the native to diazepam in the management of stiff-man syndrome hour, adjusted according to response to a maximum of 1 elderly) at night for 4 nights gradually increased over 2 60 micrograms/kg per hour. -

(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

A Coniparative Evaluation of Operating Conditions for the Electrochemical Bromination and Chlorination of Succinimide
Bulletin of Electrochemistry 16 (12) December 2000, pp 544-550 0256-1654/2000/$ 3-50 © 2000 CECRI • A CONIPARATIVE EVALUATION OF OPERATING CONDITIONS FOR THE ELECTROCHEMICAL BROMINATION AND CHLORINATION OF SUCCINIMIDE S KRISHNAMOORTHY, R KANAKAM SRINIVASAN AND M NOEL Central Electrochemical Research Institute, Karaikudi 630 006. INDIA [Received: 20 September 1998 Accepted: 19 September 2000] Platinum was found to be the electrode of choice for both electrochlorination and bromination of succinimide leading to the formation of N chloro succinimide (NCS) and N bromo succinimide (NBS) respectively. Because of the competitive processes 150% and 285% of theoretical charge has to be passed for bromination and chlorination respectively. 12% (w!v) of succinimide could be used for bromination while only 2% (w!v) of this reactant is optimum for chlorination. Optimum conditions have been evaluated for the process. A maximum yield of 55% and 88% could be achieved for bromination and chlorination of succinimide respectively. Keywords: •Chlorination, bromination succinimide INTRODUCTION A thermostated closed glass vessel with provIsions for working electrode (platinum, graphite, TSIA and GSLD) Probably because of the facile bromination [1-4] and [14-15], counter electrode, platinum (4 x 3 ems) chlorination [5-9] of succinimide (SI) by chemical means, thermometer and a glass stirrer was used as the the corresponding electrohalogenation process have not preparative cell. received considerable attention. A brief report on the After completion of electrolysis, NCS and NBS are preparation of N- bromosuccinimide (NBS) in aqueous separated by filtration and washed with a cold (275 K) sodium bromide solution on platinum electrode is saturated solution of the corresponding N-halosuccinimide.